Organic Fouling in Forward Osmosis: A Comprehensive Review
Abstract
:1. Introduction
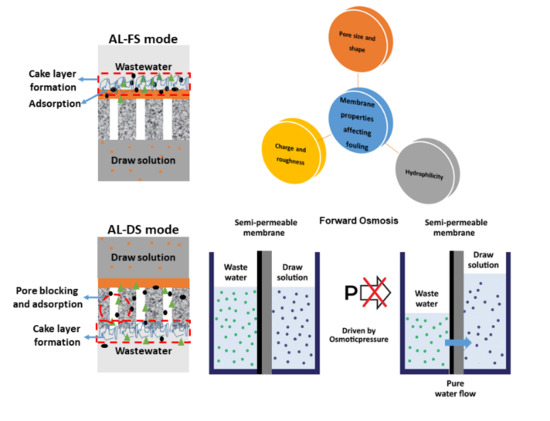
2. Fundamentals of the FO Separation
- The biggest advantage of FO in treating wastewater is its low fouling propensity. Wastewater has low osmotic pressure than seawater but has higher fouling propensity. Therefore, FO is ideal for treating complex wastewaters [60].
- Recently, a study successfully adapted FO to concentrate radioactive liquid waste generated in hospitals. FO successfully rejected natural and radioactive iodine at a rate of 99.85%, which is higher than 99.7% for Ultrafiltration (UF) and Reverse osmosis (RO) reported in the literature [61].
- An integrated forward osmosis system can treat dewatered construction water (DCW) for reuse or for discharging into the sea, which reduces the adverse impacts of DCW on the environment if discharged to the sea directly [62].
- FO combined with electrochemical oxidation can reject more than 98% of antibiotics from pharmaceutical wastewater [66].
3. Organic Fouling in the FO Process
3.1. The Adsorption Model for Predicting Permeate Flux Decline in the FO Process Due to Organics
3.2. Characteristics of Organic Foulants
3.3. Model Organic Foulants Used in the Forward Osmosis Process
3.4. Characteristics of Organic Foulants in Real Wastewater
4. Organic Fouling Mitigation with Pre-Treatment
5. Cleaning Protocols for Fouled FO Membranes
5.1. Physical Cleaning
5.2. Chemical Cleaning
5.3. Physio-Chemical Cleaning
5.4. Biological/Biochemical Cleaning
5.5. Factors Influencing Cleaning Efficiency
6. Future Outlook and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, T.-S.; Luo, L.; Wan, C.F.; Cui, Y.; Amy, G. What is next for forward osmosis (FO) and pressure retarded osmosis (PRO). Sep. Purif. Technol. 2015, 156, 856–860. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, M.; Yadav, S.; Hung, W.-S.; Lai, J.-Y. One-pot eco-friendly synthesis of edge-carboxylate graphene via dry ball milling for enhanced removal of acid and basic dyes from single or mixed aqueous solution. J. Cleaner Prod. 2020, 121498. [Google Scholar] [CrossRef]
- Meena, N.K.; Nimbalkar, S.; Fatahi, B.; Yang, G. Effects of soil arching on behavior of pile-supported railway embankment: 2D FEM approach. Comput. Geotech. 2020, 123, 103601. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Y.; Bai, J.; Li, J.; Wang, J.; Li, L.; Zhou, T.; Chen, S.; Rahim, M.; Zhou, B. Efficient denitrification and removal of natural organic matter, emerging pollutants simultaneously for RO concentrate based on photoelectrocatalytic radical reaction. Sep. Purif. Technol. 2020, 234, 116032. [Google Scholar] [CrossRef]
- Li, C.; Song, C.; Tao, P.; Sun, M.; Pan, Z.; Wang, T.; Shao, M. Enhanced separation performance of coal-based carbon membranes coupled with an electric field for oily wastewater treatment. Sep. Purif. Technol. 2016, 168, 47–56. [Google Scholar] [CrossRef]
- Jyothi, M.; Yadav, S.; Balakrishna, G. Effective recovery of acids from egg waste incorporated PSf membranes: A step towards sustainable development. J. Membr. Sci. 2018, 549, 227–235. [Google Scholar] [CrossRef]
- Akamatsu, K.; Kagami, Y.; Nakao, S.-I. Effect of BSA and sodium alginate adsorption on decline of filtrate flux through polyethylene microfiltration membranes. J. Membr. Sci. 2020, 594, 117469. [Google Scholar] [CrossRef]
- Bartels, C.; Wilf, M.; Casey, W.; Campbell, J. New generation of low fouling nanofiltration membranes. Desalination 2008, 221, 158–167. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Guo, Y.; Wang, C.; Hu, Z.; Zhang, C. High-hydrophilic and salt rejecting PA-g/co-PVP RO membrane via bionic sand-fixing grass for pharmaceutical wastewater treatment. Chem. Eng. J. 2019, 357, 269–279. [Google Scholar] [CrossRef]
- Racar, M.; Dolar, D.; Špehar, A.; Košutić, K. Application of UF/NF/RO membranes for treatment and reuse of rendering plant wastewater. Process Saf. Environ. Prot. 2017, 105, 386–392. [Google Scholar] [CrossRef]
- Okamoto, Y.; Lienhard, J.H. How RO membrane permeability and other performance factors affect process cost and energy use: A review. Desalination 2019, 470, 114064. [Google Scholar] [CrossRef]
- Yadav, S.; Saleem, H.; Ibrar, I.; Naji, O.; Hawari, A.A.; Alanezi, A.A.; Zaidi, S.J.; Altaee, A.; Zhou, J. Recent developments in forward osmosis membranes using carbon-based nanomaterials. Desalination 2020, 482, 114375. [Google Scholar] [CrossRef]
- Shon, H.; Vigneswaran, S.; Kim, I.S.; Cho, J.; Ngo, H. Fouling of ultrafiltration membrane by effluent organic matter: A detailed characterization using different organic fractions in wastewater. J. Membr. Sci. 2006, 278, 232–238. [Google Scholar] [CrossRef]
- Hancock, N.T.; Xu, P.; Heil, D.M.; Bellona, C.; Cath, T.Y. Comprehensive bench-and pilot-scale investigation of trace organic compounds rejection by forward osmosis. Environ. Sci. Technol. 2011, 45, 8483–8490. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Moody, C. Drinking water from sea water by forward osmosis. Desalination 1976, 18, 297–306. [Google Scholar] [CrossRef]
- Tow, E.W.; Warsinger, D.M.; Trueworthy, A.M.; Swaminathan, J.; Thiel, G.P.; Zubair, S.M.; Myerson, A.S. Comparison of fouling propensity between reverse osmosis, forward osmosis, and membrane distillation. J. Membr. Sci. 2018, 556, 352–364. [Google Scholar] [CrossRef] [Green Version]
- Bamaga, O.; Yokochi, A.; Zabara, B.; Babaqi, A. Hybrid FO/RO desalination system: Preliminary assessment of osmotic energy recovery and designs of new FO membrane module configurations. Desalination 2011, 268, 163–169. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Cornelissen, E.R.; Harmsen, D.J.; Post, J.W.; Lampi, K.; Ramaekers, H.; Rietveld, L.C.; Roest, K. Water recovery from sewage using forward osmosis. Water Sci. Technol. 2011, 64, 1443–1449. [Google Scholar] [CrossRef]
- Iskander, S.M.; Zou, S.; Brazil, B.; Novak, J.T.; He, Z. Energy consumption by forward osmosis treatment of landfill leachate for water recovery. Waste Manag. 2017, 63, 284–291. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z.; Zhu, C.; Wang, Q.; Tang, J.; Wu, Z. A forward osmosis membrane system for the post-treatment of MBR-treated landfill leachate. J. Membr. Sci. 2014, 471, 192–200. [Google Scholar] [CrossRef]
- Yang, S.; Gao, B.; Jang, A.; Kyong Shon, H.; Yue, Q. Municipal wastewater treatment by forward osmosis using seawater concentrate as draw solution. Chemosphere 2019, 237, 124485. [Google Scholar] [CrossRef] [PubMed]
- Lutchmiah, K.; Verliefde, A.; Roest, K.; Rietveld, L.C.; Cornelissen, E. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Phuntsho, S.; Shon, H.K.; Hong, S.; Lee, S.; Vigneswaran, S.; Kandasamy, J. Fertiliser drawn forward osmosis desalination: The concept, performance and limitations for fertigation. Rev. Environ. Sci. Bio/Technol. 2012, 11, 147–168. [Google Scholar] [CrossRef]
- Korenak, J.; Basu, S.; Balakrishnan, M.; Hélix-Nielsen, C.; Petrinic, I. Forward osmosis in wastewater treatment processes. Acta Chim. Slov. 2017, 64, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Ibrar, I.; Naji, O.; Sharif, A.; Malekizadeh, A.; Alhawari, A.; Alanezi, A.A.; Altaee, A. A review of fouling mechanisms, control strategies and real-time fouling monitoring techniques in forward osmosis. Water 2019, 11, 695. [Google Scholar] [CrossRef] [Green Version]
- Amy, G. Fundamental understanding of organic matter fouling of membranes. Desalination 2008, 231, 44–51. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Goh, K.; Li, X.; Setiawan, L.; Wang, R. Membranes and processes for forward osmosis-based desalination: Recent advances and future prospects. Desalination 2018, 434, 81–99. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Chen, S.-S.; Jain, S.; Nguyen, H.T.; Ray, S.S.; Ngo, H.H.; Guo, W.; Lam, N.T.; Duong, H.C. Exploration of an innovative draw solution for a forward osmosis-membrane distillation desalination process. Environ. Sci. Pollut. Res. 2018, 25, 5203–5211. [Google Scholar] [CrossRef]
- Bao, X.; Wu, Q.; Shi, W.; Wang, W.; Yu, H.; Zhu, Z.; Zhang, X.; Zhang, Z.; Zhang, R.; Cui, F. Polyamidoamine dendrimer grafted forward osmosis membrane with superior ammonia selectivity and robust antifouling capacity for domestic wastewater concentration. Water Res. 2019, 153, 1–10. [Google Scholar] [CrossRef]
- Nagy, E. Basic Equations of Mass Transport Through a Membrane Layer; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Zhang, S.; Wang, K.Y.; Chung, T.-S.; Chen, H.; Jean, Y.; Amy, G. Well-constructed cellulose acetate membranes for forward osmosis: Minimized internal concentration polarization with an ultra-thin selective layer. J. Membr. Sci. 2010, 360, 522–535. [Google Scholar] [CrossRef]
- Alshwairekh, A.M.; Alghafis, A.A.; Alwatban, A.M.; Alqsair, U.F.; Oztekin, A. The effects of membrane and channel corrugations in forward osmosis membrane modules–Numerical analyses. Desalination 2019, 460, 41–55. [Google Scholar] [CrossRef]
- Li, J.-Y.; Ni, Z.-Y.; Zhou, Z.-Y.; Hu, Y.-X.; Xu, X.-H.; Cheng, L.-H. Membrane fouling of forward osmosis in dewatering of soluble algal products: Comparison of TFC and CTA membranes. J. Membr. Sci. 2018, 552, 213–221. [Google Scholar] [CrossRef]
- Linares, R.V.; Li, Z.; Sarp, S.; Bucs, S.S.; Amy, G.; Vrouwenvelder, J.S. Forward osmosis niches in seawater desalination and wastewater reuse. Water Res. 2014, 66, 122–139. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; McCutcheon, J.R. A new commercial thin film composite membrane for forward osmosis. Desalination 2014, 343, 187–193. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, J.; Tang, J.; Wang, X.; Wu, Z. A pilot-scale forward osmosis membrane system for concentrating low-strength municipal wastewater: Performance and implications. Sci. Rep. 2016, 6, 21653. [Google Scholar] [CrossRef] [Green Version]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.-M.; Chen, G.; Chung, W.-J.; Shon, H.K. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Yu, Y.; Seo, S.; Kim, I.-C.; Lee, S. Nanoporous polyethersulfone (PES) membrane with enhanced flux applied in forward osmosis process. J. Membr. Sci. 2011, 375, 63–68. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Lang, W.-Z.; Wang, Y. Improved performance of thin-film composite membrane with PVDF/PFSA substrate for forward osmosis process. J. Membr. Sci. 2017, 535, 188–199. [Google Scholar] [CrossRef]
- Qin, D.; Liu, Z.; Sun, D.D.; Song, X.; Bai, H. A new nanocomposite forward osmosis membrane custom-designed for treating shale gas wastewater. Sci. Rep. 2015, 5, 14530. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Soontarapa, K.; Jyothi, M.; Padaki, M.; Balakrishna, R.G.; Lai, J.-Y. Supplementing multi-functional groups to polysulfone membranes using Azadirachta indica leaves powder for effective and highly selective acid recovery. J. Hazard. Mater. 2019, 369, 1–8. [Google Scholar] [CrossRef]
- Shahabi, S.S.; Azizi, N.; Vatanpour, V. Synthesis and characterization of novel g-C3N4 modified thin film nanocomposite reverse osmosis membranes to enhance desalination performance and fouling resistance. Sep. Purif. Technol. 2019, 215, 430–440. [Google Scholar] [CrossRef]
- Tian, M.; Wang, Y.-N.; Wang, R.; Fane, A.G. Synthesis and characterization of thin film nanocomposite forward osmosis membranes supported by silica nanoparticle incorporated nanofibrous substrate. Desalination 2017, 401, 142–150. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A.F. A novel thin film composite forward osmosis membrane prepared from PSf–TiO2 nanocomposite substrate for water desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Liao, R.; Tang, C.Y. Zeolite-polyamide thin film nanocomposite membranes: Towards enhanced performance for forward osmosis. J. Membr. Sci. 2012, 405, 149–157. [Google Scholar] [CrossRef]
- Zou, S.; Smith, E.D.; Lin, S.; Martin, S.M.; He, Z. Mitigation of bidirectional solute flux in forward osmosis via membrane surface coating of zwitterion functionalized carbon nanotubes. Environ. Int. 2019, 131, 104970. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Kim, C.-M.; Song, J.-h.; Ki, H.; Ham, M.-H.; Kim, I.S. Enhanced desalination performance of forward osmosis membranes based on reduced graphene oxide laminates coated with hydrophilic polydopamine. Carbon 2017, 117, 293–300. [Google Scholar] [CrossRef]
- Wu, X.; Field, R.W.; Wu, J.J.; Zhang, K. Polyvinylpyrrolidone modified graphene oxide as a modifier for thin film composite forward osmosis membranes. J. Membr. Sci. 2017, 540, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Saleem, H.; Trabzon, L.; Kilic, A.; Zaidi, S.J. Recent advances in nanofibrous membranes: Production and applications in water treatment and desalination. Desalination 2020, 478, 114178. [Google Scholar] [CrossRef]
- Song, X.; Liu, Z.; Sun, D.D. Nano gives the answer: Breaking the bottleneck of internal concentration polarization with a nanofiber composite forward osmosis membrane for a high water production rate. Adv. Mater. 2011, 23, 3256–3260. [Google Scholar] [CrossRef]
- Pardeshi, P.M.; Mungray, A.K.; Mungray, A.A. Polyvinyl chloride and layered double hydroxide composite as a novel substrate material for the forward osmosis membrane. Desalination 2017, 421, 149–159. [Google Scholar] [CrossRef]
- Vu, M.T.; Ansari, A.J.; Hai, F.I.; Nghiem, L.D. Performance of a seawater-driven forward osmosis process for pre-concentrating digested sludge centrate: Organic enrichment and membrane fouling. Environ. Sci. Water Res. Technol. 2018, 4, 1047–1056. [Google Scholar] [CrossRef]
- Vu, M.T.; Price, W.E.; He, T.; Zhang, X.; Nghiem, L.D. Seawater-driven forward osmosis for pre-concentrating nutrients in digested sludge centrate. J. Environ. Manag. 2019, 247, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Selection of inorganic-based draw solutions for forward osmosis applications. J. Membr. Sci. 2010, 364, 233–241. [Google Scholar] [CrossRef]
- Johnson, D.J.; Suwaileh, W.A.; Mohammed, A.W.; Hilal, N. Osmotic’s potential: An overview of draw solutes for forward osmosis. Desalination 2018, 434, 100–120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, J.; Cui, H.; Li, H.; Yang, F. Forward osmosis using electric-responsive polymer hydrogels as draw agents: Influence of freezing–thawing cycles, voltage, feed solutions on process performance. Chem. Eng. J. 2015, 259, 814–819. [Google Scholar] [CrossRef]
- Phuntsho, S.; Shon, H.K.; Hong, S.; Lee, S.; Vigneswaran, S. A novel low energy fertilizer driven forward osmosis desalination for direct fertigation: Evaluating the performance of fertilizer draw solutions. J. Membr. Sci. 2011, 375, 172–181. [Google Scholar] [CrossRef]
- Korenak, J.; Hélix-Nielsen, C.; Bukšek, H.; Petrinić, I. Efficiency and economic feasibility of forward osmosis in textile wastewater treatment. J. Clean. Prod. 2019, 210, 1483–1495. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.; Park, J.; Shon, H.K.; Hong, S. Treatment of medical radioactive liquid waste using Forward Osmosis (FO) membrane process. J. Membr. Sci. 2018, 556, 238–247. [Google Scholar] [CrossRef]
- Hawari, A.H.; Al-Qahoumi, A.; Ltaief, A.; Zaidi, S.; Altaee, A. Dilution of seawater using dewatered construction water in a hybrid forward osmosis system. J. Clean. Prod. 2018, 195, 365–373. [Google Scholar] [CrossRef]
- Dutta, S.; Nath, K. Feasibility of forward osmosis using ultra low pressure RO membrane and Glauber salt as draw solute for wastewater treatment. J. Environ. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Lee, W.J.; Goh, P.S.; Lau, W.J.; Ong, C.S.; Ismail, A.F. Antifouling zwitterion embedded forward osmosis thin film composite membrane for highly concentrated oily wastewater treatment. Sep. Purif. Technol. 2018. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Wang, J. Removal of Cs(I) from simulated radioactive wastewater by three forward osmosis membranes. Chem. Eng. J. 2018, 344, 353–362. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Feng, Y.; Shen, C.; Yang, F. Integrating electrochemical oxidation into forward osmosis process for removal of trace antibiotics in wastewater. J. Hazard. Mater. 2015, 296, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Ang, W.L.; Mohammad, A.W.; Johnson, D.; Hilal, N. Forward osmosis research trends in desalination and wastewater treatment: A review of research trends over the past decade. J. Water Process Eng. 2019, 31, 100886. [Google Scholar] [CrossRef]
- Aftab, B.; Ok, Y.S.; Cho, J.; Hur, J. Targeted removal of organic foulants in landfill leachate in forward osmosis system integrated with biochar/activated carbon treatment. Water Res. 2019, 160, 217–227. [Google Scholar] [CrossRef]
- Lee, D.-J.; Hsieh, M.-H. Forward osmosis membrane processes for wastewater bioremediation: Research needs. Bioresour. Technol. 2019, 121795. [Google Scholar] [CrossRef]
- Rudolph, G.; Virtanen, T.; Ferrando, M.; Güell, C.; Lipnizki, F.; Kallioinen, M. A review of in situ real-time monitoring techniques for membrane fouling in the biotechnology, biorefinery and food sectors. J. Membr. Sci. 2019, 117221. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Springer Science & Business Media: Cham, Switzerland, 2012. [Google Scholar]
- Baudequin, C.; Mai, Z.; Rakib, M.; Deguerry, I.; Severac, R.; Pabon, M.; Couallier, E. Removal of fluorinated surfactants by reverse osmosis–role of surfactants in membrane fouling. J. Membr. Sci. 2014, 458, 111–119. [Google Scholar] [CrossRef]
- Sioutopoulos, D.; Goudoulas, T.; Kastrinakis, E.; Nychas, S.; Karabelas, A. Rheological and permeability characteristics of alginate fouling layers developing on reverse osmosis membranes during desalination. J. Membr. Sci. 2013, 434, 74–84. [Google Scholar] [CrossRef]
- Braeken, L.; Van der Bruggen, B.; Vandecasteele, C. Flux Decline in Nanofiltration Due to Adsorption of Dissolved Organic Compounds: Model Prediction of Time Dependency. J. Phys. Chem. B 2006, 110, 2957–2962. [Google Scholar] [CrossRef]
- Li, H.; Xia, H.; Mei, Y. Modeling organic fouling of reverse osmosis membrane: From adsorption to fouling layer formation. Desalination 2016, 386, 25–31. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; Elimelech, M. Influence of concentrative and dilutive internal concentration polarization on flux behavior in forward osmosis. J. Membr. Sci. 2006, 284, 237–247. [Google Scholar] [CrossRef]
- Yip, N.Y.; Tiraferri, A.; Phillip, W.A.; Schiffman, J.D.; Hoover, L.A.; Kim, Y.C.; Elimelech, M. Thin-film composite pressure retarded osmosis membranes for sustainable power generation from salinity gradients. Environ. Sci. Technol. 2011, 45, 4360–4369. [Google Scholar] [CrossRef] [PubMed]
- Her, N.; Amy, G.; McKnight, D.; Sohn, J.; Yoon, Y. Characterization of DOM as a function of MW by fluorescence EEM and HPLC-SEC using UVA, DOC, and fluorescence detection. Water Res. 2003, 37, 4295–4303. [Google Scholar] [CrossRef]
- Frimmel, F.H.; Abbt-Braun, G.; Heumann, K.G.; Hock, B.; Lüdemann, H.-D.; Spiteller, M. Refractory Organic Substances in the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Van Geluwe, S.; Braeken, L.; Van der Bruggen, B. Ozone oxidation for the alleviation of membrane fouling by natural organic matter: A review. Water Res. 2011, 45, 3551–3570. [Google Scholar] [CrossRef] [Green Version]
- McDonald, S.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Analytical chemistry of freshwater humic substances. Anal. Chim. Acta 2004, 527, 105–124. [Google Scholar] [CrossRef]
- Hessen, D.; Tranvik, L.J. Aquatic Humic Substances: Ecology and Biogeochemistry; Springer Science & Business Media: Cham, Switzerland, 2013; Volume 133. [Google Scholar]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward osmosis membranes and processes: A comprehensive review of research trends and future outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Gwak, G.; Kim, D.I.; Hong, S. New industrial application of forward osmosis (FO): Precious metal recovery from printed circuit board (PCB) plant wastewater. J. Membr. Sci. 2018, 552, 234–242. [Google Scholar] [CrossRef]
- Coday, B.D.; Xu, P.; Beaudry, E.G.; Herron, J.; Lampi, K.; Hancock, N.T.; Cath, T.Y. The sweet spot of forward osmosis: Treatment of produced water, drilling wastewater, and other complex and difficult liquid streams. Desalination 2014, 333, 23–35. [Google Scholar] [CrossRef]
- Kim, J.E.; Phuntsho, S.; Ali, S.M.; Choi, J.Y.; Shon, H.K. Forward osmosis membrane modular configurations for osmotic dilution of seawater by forward osmosis and reverse osmosis hybrid system. Water Res. 2018, 128, 183–192. [Google Scholar] [CrossRef]
- Ali, S.M.; Kim, J.E.; Phuntsho, S.; Jang, A.; Choi, J.Y.; Shon, H.K. Forward osmosis system analysis for optimum design and operating conditions. Water Res. 2018, 145, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Ly, Q.V.; Hu, Y.; Li, J.; Cho, J.; Hur, J. Characteristics and influencing factors of organic fouling in forward osmosis operation for wastewater applications: A comprehensive review. Environ. Int. 2019, 129, 164–184. [Google Scholar] [CrossRef] [PubMed]
- Boo, C.; Lee, S.; Elimelech, M.; Meng, Z.; Hong, S. Colloidal fouling in forward osmosis: Role of reverse salt diffusion. J. Membr. Sci. 2012, 390, 277–284. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Chemical and physical aspects of organic fouling of forward osmosis membranes. J. Membr. Sci. 2008, 320, 292–302. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.-N.; Wei, J.; Tang, C.Y. Organic fouling of thin-film composite polyamide and cellulose triacetate forward osmosis membranes by oppositely charged macromolecules. Water Res. 2013, 47, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Motsa, M.M.; Mamba, B.B.; Verliefde, A.R. Forward osmosis membrane performance during simulated wastewater reclamation: Fouling mechanisms and fouling layer properties. J. Water Process Eng. 2018, 23, 109–118. [Google Scholar] [CrossRef]
- Filloux, E.; Gallard, H.; Croue, J.-P. Identification of effluent organic matter fractions responsible for low-pressure membrane fouling. Water Res. 2012, 46, 5531–5540. [Google Scholar] [CrossRef]
- Aosai, D.; Saeki, D.; Iwatsuki, T.; Matsuyama, H. Efficient condensation of organic colloids in deep groundwater using surface-modified nanofiltration membranes under optimized hydrodynamic conditions. Colloids Surf. A Physicochem. Eng. Asp. 2016, 495, 68–78. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Wang, L.; Zhang, W.; Chu, H.; Dong, B. Forward osmosis filtration for removal of organic foulants: Effects of combined tannic and alginic acids. Water Res. 2016, 91, 251–263. [Google Scholar] [CrossRef]
- Hong, S.; Elimelech, M. Chemical and physical aspects of natural organic matter (NOM) fouling of nanofiltration membranes. J. Membr. Sci. 1997, 132, 159–181. [Google Scholar] [CrossRef]
- Seidel, A.; Elimelech, M. Coupling between chemical and physical interactions in natural organic matter (NOM) fouling of nanofiltration membranes: Implications for fouling control. J. Membr. Sci. 2002, 203, 245–255. [Google Scholar] [CrossRef]
- Lee, S.; Boo, C.; Elimelech, M.; Hong, S. Comparison of fouling behavior in forward osmosis (FO) and reverse osmosis (RO). J. Membr. Sci. 2010, 365, 34–39. [Google Scholar] [CrossRef]
- Abdikheibari, S.; Dumée, L.F.; Jegatheesan, V.; Mustafa, Z.; Le-Clech, P.; Lei, W.; Baskaran, K. Natural organic matter removal and fouling resistance properties of a boron nitride nanosheet-functionalized thin film nanocomposite membrane and its impact on permeate chlorine demand. J. Water Process Eng. 2020, 34, 101160. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, P.; Xu, S.; Koroleva, M.; Zhang, S.; Si, S.; Jin, Z.G. Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Parida, V.; Ng, H.Y. Forward osmosis organic fouling: Effects of organic loading, calcium and membrane orientation. Desalination 2013, 312, 88–98. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Organic fouling of forward osmosis membranes: Fouling reversibility and cleaning without chemical reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Mazlan, N.M.; Marchetti, P.; Maples, H.; Gu, B.; Karan, S.; Bismarck, A.; Livingston, A.G. Organic fouling behaviour of structurally and chemically different forward osmosis membranes–a study of cellulose triacetate and thin film composite membranes. J. Membr. Sci. 2016, 520, 247–261. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.-T.; Lee, C.; Field, R.W.; Kim, I.S. Insight into organic fouling behavior in polyamide thin-film composite forward osmosis membrane: Critical flux and its impact on the economics of water reclamation. J. Membr. Sci. 2020, 606, 118118. [Google Scholar] [CrossRef]
- She, Q.; Wong, Y.K.W.; Zhao, S.; Tang, C.Y. Organic fouling in pressure retarded osmosis: Experiments, mechanisms and implications. J. Membr. Sci. 2013, 428, 181–189. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L.; Dong, B. Effects of tannic acid on membrane fouling and membrane cleaning in forward osmosis. Water Sci. Technol. 2017, 76, 3160–3170. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, L.; Tang, C. Investigation on removing recalcitrant toxic organic polluters in coking wastewater by forward osmosis. Chin. J. Chem. Eng. 2020, 28, 122–135. [Google Scholar] [CrossRef]
- Wang, S.; Amornwittawat, N.; Banatlao, J.; Chung, M.; Kao, Y.; Wen, X. Hofmeister effects of common monovalent salts on the beetle antifreeze protein activity. J. Phys. Chem. B 2009, 113, 13891–13894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, C.M.; Li, X.-Y.; Li, Q. The combined colloid-organic fouling on nanofiltration membrane for wastewater treatment and reuse. Sep. Sci. Technol. 2010, 45, 935–940. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Nasseri, S.; Ulbricht, M. Fouling effects of humic and alginic acids in nanofiltration and influence of solution composition. Desalination 2010, 250, 688–692. [Google Scholar] [CrossRef]
- Balkenov, A.; Anuarbek, A.; Satayeva, A.; Kim, J.; Inglezakis, V.; Arkhangelsky, E. Complex organic fouling and effect of silver nanoparticles on aquaporin forward osmosis membranes. J. Water Process Eng. 2020, 34, 101177. [Google Scholar] [CrossRef]
- Kim, Y.; Elimelech, M.; Shon, H.K.; Hong, S. Combined organic and colloidal fouling in forward osmosis: Fouling reversibility and the role of applied pressure. J. Membr. Sci. 2014, 460, 206–212. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Shu, L.; Jegatheesan, V.; Bhuiyan, M.A. Effect of the coagulation/persulfate pre-treatment to mitigate organic fouling in the forward osmosis of municipal wastewater treatment. J. Environ. Manag. 2019, 249, 109394. [Google Scholar] [CrossRef]
- Aftab, B.; Cho, J.; Hur, J. Intermittent osmotic relaxation: A strategy for organic fouling mitigation in a forward osmosis system treating landfill leachate. Desalination 2020, 482, 114406. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Hai, F.I.; Roddick, F.A. Ultraviolet/persulfate pre-treatment for organic fouling mitigation of forward osmosis membrane: Possible application in nutrient mining from dairy wastewater. Sep. Purif. Technol. 2019, 217, 215–220. [Google Scholar] [CrossRef]
- Im, S.-J.; Jeong, G.; Jeong, S.; Cho, J.; Jang, A. Fouling and transport of organic matter in cellulose triacetate forward-osmosis membrane for wastewater reuse and seawater desalination. Chem. Eng. J. 2020, 384, 123341. [Google Scholar] [CrossRef]
- Sun, F.; Lu, D.; Ho, J.S.; Chong, T.H.; Zhou, Y. Mitigation of membrane fouling in a seawater-driven forward osmosis system for waste activated sludge thickening. J. Clean. Prod. 2019, 241, 118373. [Google Scholar] [CrossRef]
- Ye, J.; Zhou, Q.; Zhang, X.; Hu, Q. Microalgal dewatering using a polyamide thin film composite forward osmosis membrane and fouling mitigation. Algal Res. 2018, 31, 421–429. [Google Scholar] [CrossRef]
- Akther, N.; Yuan, Z.; Chen, Y.; Lim, S.; Phuntsho, S.; Ghaffour, N.; Matsuyama, H.; Shon, H. Influence of graphene oxide lateral size on the properties and performances of forward osmosis membrane. Desalination 2020, 484, 114421. [Google Scholar] [CrossRef]
- Ibrar, I.; Altaee, A.; Zhou, J.L.; Naji, O.; Khanafer, D. Challenges and potentials of forward osmosis process in the treatment of wastewater. Crit. Rev. Environ. Sci. Technol. 2019, 1–45. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.; Matsuura, T.; Hilal, N.; Ismail, A. The potential of thin film nanocomposite membrane in reducing organic fouling in forward osmosis process. Desalination 2014, 348, 82–88. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, B. Effects of organic macromolecular conditioning on gypsum scaling of forward osmosis membranes. J. Membr. Sci. 2014, 450, 153–161. [Google Scholar] [CrossRef]
- Carroll, T.; King, S.; Gray, S.; Bolto, B.A.; Booker, N. The fouling of microfiltration membranes by NOM after coagulation treatment. Water Res. 2000, 34, 2861–2868. [Google Scholar] [CrossRef]
- Blume, T.; Neis, U. Improved wastewater disinfection by ultrasonic pre-treatment. Ultrason. Sonochemistry 2004, 11, 333–336. [Google Scholar] [CrossRef]
- Suarez, S.; Lema, J.M.; Omil, F. Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresour. Technol. 2009, 100, 2138–2146. [Google Scholar] [CrossRef]
- Hancock, N.T.; Black, N.D.; Cath, T.Y. A comparative life cycle assessment of hybrid osmotic dilution desalination and established seawater desalination and wastewater reclamation processes. Water Res. 2012, 46, 1145–1154. [Google Scholar] [CrossRef]
- Engelhardt, S.; Vogel, J.; Duirk, S.E.; Moore, F.B.; Barton, H.A. Assessment of urea hydrolysis as a pretreatment strategy to improve total nitrogen rejection from urine using aquaporin-based membranes in forward osmosis. J. Water Process Eng. 2020, 34, 101135. [Google Scholar] [CrossRef]
- Al Hawli, B.; Benamor, A.; Hawari, A.A. A hybrid electro-coagulation/forward osmosis system for treatment of produced water. Chem. Eng. Process. Process Intensif. 2019, 143, 107621. [Google Scholar] [CrossRef]
- Jamil, S.; Loganathan, P.; Kazner, C.; Vigneswaran, S. Forward osmosis treatment for volume minimisation of reverse osmosis concentrate from a water reclamation plant and removal of organic micropollutants. Desalination 2015, 372, 32–38. [Google Scholar] [CrossRef]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.-L.; Han, Z.-S.; Li, G.-B. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Le-Clech, P.; Chen, V.; Fane, T.A. Fouling in membrane bioreactors used in wastewater treatment. J. Membr. Sci. 2006, 284, 17–53. [Google Scholar] [CrossRef]
- Ndinisa, N.; Fane, A.; Wiley, D.; Fletcher, D. Fouling control in a submerged flat sheet membrane system: Part II—Two-phase flow characterization and CFD simulations. Sep. Sci. Technol. 2006, 41, 1411–1445. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Wicaksana, F.; Chou, S.; Al-Rabiah, A.A.; Al-Zahrani, S.M.; Wang, R. Effects of scaling and cleaning on the performance of forward osmosis hollow fiber membranes. J. Membr. Sci. 2012, 415, 101–108. [Google Scholar] [CrossRef]
- Yip, N.Y.; Elimelech, M. Influence of natural organic matter fouling and osmotic backwash on pressure retarded osmosis energy production from natural salinity gradients. Environ. Sci. Technol. 2013, 47, 12607–12616. [Google Scholar] [CrossRef]
- Holloway, R.W.; Childress, A.E.; Dennett, K.E.; Cath, T.Y. Forward osmosis for concentration of anaerobic digester centrate. Water Res. 2007, 41, 4005–4014. [Google Scholar] [CrossRef]
- Hickenbottom, K.L.; Hancock, N.T.; Hutchings, N.R.; Appleton, E.W.; Beaudry, E.G.; Xu, P.; Cath, T.Y. Forward osmosis treatment of drilling mud and fracturing wastewater from oil and gas operations. Desalination 2013, 312, 60–66. [Google Scholar] [CrossRef]
- Lay, W.C.; Zhang, J.; Tang, C.; Wang, R.; Liu, Y.; Fane, A.G. Factors affecting flux performance of forward osmosis systems. J. Membr. Sci. 2012, 394, 151–168. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Marchand, E.A.; Childress, A.E. The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes. Desalination 2009, 239, 10–21. [Google Scholar] [CrossRef]
- Hancock, N.T.; Xu, P.; Roby, M.J.; Gomez, J.D.; Cath, T.Y. Towards direct potable reuse with forward osmosis: Technical assessment of long-term process performance at the pilot scale. J. Membr. Sci. 2013, 445, 34–46. [Google Scholar] [CrossRef]
- Linares, R.V.; Li, Z.; Abu-Ghdaib, M.; Wei, C.-H.; Amy, G.; Vrouwenvelder, J.S. Water harvesting from municipal wastewater via osmotic gradient: An evaluation of process performance. J. Membr. Sci. 2013, 447, 50–56. [Google Scholar] [CrossRef]
- Tragardh, G. Membrane cleaning. Desalination 1989, 71, 325–335. [Google Scholar] [CrossRef]
- Maartens, A.; Jacobs, E.; Swart, P. UF of pulp and paper effluent: Membrane fouling-prevention and cleaning. J. Membr. Sci. 2002, 209, 81–92. [Google Scholar] [CrossRef]
- Zondervan, E.; Roffel, B. Evaluation of different cleaning agents used for cleaning ultra filtration membranes fouled by surface water. J. Membr. Sci. 2007, 304, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Weis, A.; Bird, M.R.; Nyström, M. The chemical cleaning of polymeric UF membranes fouled with spent sulphite liquor over multiple operational cycles. J. Membr. Sci. 2003, 216, 67–79. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Li, Z.; Yangali-Quintanilla, V.; Li, Q.; Amy, G. Cleaning protocol for a FO membrane fouled in wastewater reuse. Desalin. Water Treat. 2013, 51, 4821–4824. [Google Scholar] [CrossRef]
- Yoon, H.; Baek, Y.; Yu, J.; Yoon, J. Biofouling occurrence process and its control in the forward osmosis. Desalination 2013, 325, 30–36. [Google Scholar] [CrossRef]
- Linares, R.V.; Yangali-Quintanilla, V.; Li, Z.; Amy, G. NOM and TEP fouling of a forward osmosis (FO) membrane: Foulant identification and cleaning. J. Membr. Sci. 2012, 421, 217–224. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, J.; Zhu, C.; Dong, Y.; Wang, Q.; Wu, Z. Chemical cleaning protocols for thin film composite (TFC) polyamide forward osmosis membranes used for municipal wastewater treatment. J. Membr. Sci. 2015, 475, 184–192. [Google Scholar] [CrossRef]
- Qin, J.-J.; Liberman, B.; A Kekre, K.; Gossan, A. Direct osmosis for reverse osmosis fouling control: Principles, applications and recent developments. Open Chem. Eng. J. 2009, 3. [Google Scholar] [CrossRef] [Green Version]
- Ang, W.S.; Yip, N.Y.; Tiraferri, A.; Elimelech, M. Chemical cleaning of RO membranes fouled by wastewater effluent: Achieving higher efficiency with dual-step cleaning. J. Membr. Sci. 2011, 382, 100–106. [Google Scholar] [CrossRef]
- Ang, W.S.; Tiraferri, A.; Chen, K.L.; Elimelech, M. Fouling and cleaning of RO membranes fouled by mixtures of organic foulants simulating wastewater effluent. J. Membr. Sci. 2011, 376, 196–206. [Google Scholar] [CrossRef]
- Buzatu, P.; Zsirai, T.; Aerts, P.; Judd, S. Permeability and clogging in an immersed hollow fibre membrane bioreactor. J. Membr. Sci. 2012, 421, 342–348. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Tang, C.Y.; Kimura, K.; Wang, Q.; Han, X. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]
- Cai, M.; Wang, S.; Zheng, Y.; Liang, H. Effects of ultrasound on ultrafiltration of Radix astragalus extract and cleaning of fouled membrane. Sep. Purif. Technol. 2009, 68, 351–356. [Google Scholar] [CrossRef]
- Maskooki, A.; Mortazavi, S.A.; Maskooki, A. Cleaning of spiralwound ultrafiltration membranes using ultrasound and alkaline solution of EDTA. Desalination 2010, 264, 63–69. [Google Scholar] [CrossRef]
- Kwon, B.; Park, N.; Cho, J. Effect of algae on fouling and efficiency of UF membranes. Desalination 2005, 179, 203–214. [Google Scholar] [CrossRef]
- Munoz-Aguado, M.; Wiley, D.; Fane, A. Enzymatic and detergent cleaning of a polysulfone ultrafiltration membrane fouled with BSA and whey. J. Membr. Sci. 1996, 117, 175–187. [Google Scholar] [CrossRef]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Te Poele, S.; Van der Graaf, J. Enzymatic cleaning in ultrafiltration of wastewater treatment plant effluent. Desalination 2005, 179, 73–81. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Judd, S. The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Liang, H.; Gong, W.; Chen, J.; Li, G. Cleaning of fouled ultrafiltration (UF) membrane by algae during reservoir water treatment. Desalination 2008, 220, 267–272. [Google Scholar] [CrossRef]
- Al-Amoudi, A.; Lovitt, R.W. Fouling strategies and the cleaning system of NF membranes and factors affecting cleaning efficiency. J. Membr. Sci. 2007, 303, 4–28. [Google Scholar] [CrossRef]
- Porcelli, N.; Judd, S. Chemical cleaning of potable water membranes: A review. Sep. Purif. Technol. 2010, 71, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry: Part A: Structure and Mechanisms; Springer Science & Business Media: Cham, Switzerland, 2007. [Google Scholar]
- Bartlett, M.; Bird, M.; Howell, J. An experimental study for the development of a qualitative membrane cleaning model. J. Membr. Sci. 1995, 105, 147–157. [Google Scholar] [CrossRef]
- Ang, W.S.; Lee, S.; Elimelech, M. Chemical and physical aspects of cleaning of organic-fouled reverse osmosis membranes. J. Membr. Sci. 2006, 272, 198–210. [Google Scholar] [CrossRef]
- Li, T.; Law, A.W.-K.; Cetin, M.; Fane, A. Fouling control of submerged hollow fibre membranes by vibrations. J. Membr. Sci. 2013, 427, 230–239. [Google Scholar] [CrossRef]
- Chen, D.; Weavers, L.K.; Walker, H.W. Ultrasonic control of ceramic membrane fouling by particles: Effect of ultrasonic factors. Ultrason. Sonochemistry 2006, 13, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Caothien, S.; Hayes, J.; Caothuy, T.; Otoyo, T.; Ogawa, T. Membrane chemical cleaning: From art to science. Pall Corp. Port Wash. NY 2001, 11050. [Google Scholar]
- Wang, Z.; Zhang, Q.; Ma, Y.; Yang, L.; Lyu, Y.; Zhao, S. Influence of various operating conditions on cleaning efficiency in sequencing batch reactor (SBR) activated sludge process. Part III: Chemical cleaning. Desalination 2013, 325, 122–131. [Google Scholar] [CrossRef]
- Ang, W.S. Optimization of Chemical Cleaning of Organic-Fouled Reverse Osmosis Membranes; Yale University: New Haven, CT, USA, 2008. [Google Scholar]
- Wang, Z.; Meng, F.; He, X.; Zhou, Z.; Huang, L.-N.; Liang, S. Optimisation and performance of NaClO-assisted maintenance cleaning for fouling control in membrane bioreactors. Water Res. 2014, 53, 1–11. [Google Scholar] [CrossRef]
- Pavlova, S. Study on the cleaning of new ultrafiltration spiral-woundmodules to prevent membrane fouling (including biological fouling). Desalination 2005, 172, 267–270. [Google Scholar] [CrossRef]
- Allie, Z.; Jacobs, E.; Maartens, A.; Swart, P. Enzymatic cleaning of ultrafiltration membranes fouled by abattoir effluent. J. Membr. Sci. 2003, 218, 107–116. [Google Scholar] [CrossRef]




| Type of Fouling | Model Foulant | Molecular Weight ** | Properties | Reference |
|---|---|---|---|---|
| Organic | Sodium Alginate | 216.12 g/mol | Represents polysaccharides. Carboxylic groups are protonated under neutral pH. The molecular structure is not sensitive to photo excitation. | [103,104,105] |
| Organic | Bovine Serum Albumin (BSA), Humic Acid, Sodium Alginate | 66430.3 g/mol 227.17 g/mol | BSA represents proteins, while humic acid represents humic matter. | [90] |
| Organic | Alginate, humic acid, and BSA | - | Carboxylic acidities are 3.5, 3.4, and 1.0 meq/g, respectively, for the three foulants. | [99,105] |
| Organic | Humic Acid salt | - | Carboxylic groups are protonated under neutral pH conditions. | [105,106] |
| Organic | Tannic acid | 1701.19 g/mol | High tannic acid in feed solution affects FO membrane retention. Enhances internal concentration polarisation by penetrating the porous layer. | [96,107] |
| Organic | Indole | 117.15 g/mol | Nitrogen-containing heterocyclic agent found in coking wastewater. | [108] |
| Organic | Pyridine | 79.1 g/mol | Mostly found in coking wastewater. Endocrine disruptor. | [108] |
| Type of Wastewater | Membrane Used | Draw Solution | Major Findings | Ref. |
|---|---|---|---|---|
| Municipal wastewater | Thin-film composite (TFC) Porifera | 1 M Sodium chloride (NaCl) | Larger molecular organics contribute more to reversible fouling, whereas low molecular organic promotes irreversible fouling. | [114] |
| Biologically treated landfill leachate | Cellulose triacetate (CTA) HTI | 3 M NaCl | Protein and polysaccharides are major organic foulants in the landfill leachate. | [20] |
| Landfill leachate | CTA Fluid Technology Solution (FTS) | 5 M NaCl | Low molecular weight organics have more pore-penetrating ability and strong binding with the membrane surface. | [115] |
| Dairy wastewater | TFC Porifera | 1 M NaCl | No passage of building blocks, low molecular weight organics, and humic substances through the FO membrane, and these are responsible for organic fouling. | [116] |
| Secondary wastewater effluent | CTA Hydration Technology Innovations (HTI) | 0.5 M NaCl | Hydrophilic high molecular weight organics are the initial foulants on the FO membrane. | [117] |
| Waste activated sludge | TFC (Toray chemicals, Korea) | Synthetic seawater | Organic and inorganic compounds promoted fouling on the membrane. | [118] |
| Microalgae wastewater | TFC Porifera | 2 M Magnesium chloride (MgCl2) | Microalgae cells and algogenic organic matter was mainly responsible for fouling on the FO membrane. | [119] |
| Soluble algal products wastewater | CTA HTI and TFC (lab-fabricated) | 1 M NaCl, Calcium chloride (CaCl2) and MgCl2 | Fouling was more severe with CaCl2 as the draw solution. The adsorption of algal products was higher for the TFC membrane than the CTA membrane. | [34] |
| Cleaning Method | Membrane Type | Feed Solution | Draw Solution | Factors/Cleaning Condition | Cleaning Efficiency % | Flux % | Ref. |
|---|---|---|---|---|---|---|---|
| Mechanical | Vertical hollow fibres | Bentonite | 8 mm amplitude + 8 Hz frequency vibration compared to no vibration | 90 | - | [169] | |
| - | |||||||
| γ-Al2O3 ceramic | Natural water include colloidal silica | - | 3.5 cm | 100 | 60 | [170] | |
| 2.6 cm | 75 | ||||||
| 1.7 cm | 97 | ||||||
| Distance between the ultrasonic source probe and the membrane surface for 1.56 µm particles | |||||||
| Chemical | Hydrophobic Polyvinylidene fluoride (PVDF) membrane | Surface water | - | 150 | [171] | ||
| - | Oxidants and bases | 60 | |||||
| 400 ppm NaClO | 10 | ||||||
| 0.1 M NaOH | |||||||
| Cellulose acetate (CA) | 200 mg/L alginate, 50 mM NaCl, and 0.5 mM Ca2+ | (DI) water with 28 bars (400 psi) | 21 cm/s, 50 mM NaCl solution for 15 min | - | RO = 70 | [103] | |
| FO | |||||||
| 4 M NaCl | FO = 100 | ||||||
| Combined chemicals | Polyethersul-fone (PES) | Synthetic wastewater | - | NaClO + citric acid | 80% | 70–78 | [172] |
| recover membrane permeability higher than single-agent cleaning | |||||||
| Mechanical-Chemical | RO/LFC-1 | Suwannee River natural organic matter | −ethylene diamine tetraacetic acid (EDTA) 0.5 mM + pH 5.7 | - | [173] | ||
| - | 25 | ||||||
| −EDTA 0.5 mM + pH 11 for 15 min T20 °C | |||||||
| −EDTA 2 mM + pH 11 for 15 min | 43 | ||||||
| −EDTA 0.5 mM + pH 11 for 60 min | |||||||
| 100 | |||||||
| −EDTA 0.5 mM + pH 11 for 15 min T40 °C | |||||||
| 85 | |||||||
| 97 | |||||||
| −sodium dodecyl sulfate (SDS) | - | [173] | |||||
| 2 mM + pH 11 | 15 | ||||||
| −SDS 2 mM + pH 11 for 15 min | |||||||
| 18 | |||||||
| −SDS 2 mM + pH 11 for 60 min | |||||||
| −SDS 10 mM + pH 11 for 15 min | 25 | ||||||
| −SDS 10 mM + pH 11 for 60 min | |||||||
| 70 | |||||||
| 95 | |||||||
| Hollow fibre | Seeding sludge + synthetic municipal wastewater | - | 0.5 ppm NaClO solution + | - | 77 | [174] | |
| Backflush 15 min | |||||||
| Polyethersul-fone (PES) flat sheet UF | Extract of Radix astragalus (RA) | - | Ultrasound 20 kHz and 120 W + 0.1 M NaOH | - | 80 | [155] | |
| FO cellulose acetate (CA) | Sodium alginate | - | −NaCl solution at | - | 96 | [103] | |
| (10 g/L) | 8.5 cm/s for 24 h | ||||||
| −NaCl solution at | 98 | ||||||
| 21 cm/s after 15 min of rinsing | |||||||
| Biological | Ultrafiltration (UF) membrane | wastewater treatment plant | - | New enzymatic cleaning protocol (25–30 °C) | 100 | - | [160] |
| Bulgarian UF 60 Polyacrylonitrile (PAN) spiral-wound | Water from the Kamchia dam after its chlorination | Preliminary treatment of the water-chlorination or UV irradiation by low-pressure mercury- vapor lamps at a wavelength of 253.7 nm | 80 | - | [175] | ||
| Flat-sheet polysulfone (PSf) | Abattoir process | - | Pseudomonas lipase in conjunction with the identified proteases | - | −100 | [176] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, S.; Ibrar, I.; Bakly, S.; Khanafer, D.; Altaee, A.; Padmanaban, V.C.; Samal, A.K.; Hawari, A.H. Organic Fouling in Forward Osmosis: A Comprehensive Review. Water 2020, 12, 1505. https://doi.org/10.3390/w12051505
Yadav S, Ibrar I, Bakly S, Khanafer D, Altaee A, Padmanaban VC, Samal AK, Hawari AH. Organic Fouling in Forward Osmosis: A Comprehensive Review. Water. 2020; 12(5):1505. https://doi.org/10.3390/w12051505
Chicago/Turabian StyleYadav, Sudesh, Ibrar Ibrar, Salam Bakly, Daoud Khanafer, Ali Altaee, V. C. Padmanaban, Akshaya Kumar Samal, and Alaa H. Hawari. 2020. "Organic Fouling in Forward Osmosis: A Comprehensive Review" Water 12, no. 5: 1505. https://doi.org/10.3390/w12051505