Nitrogen Removal Ability and Characteristics of the Laboratory-Scale Tidal Flow Constructed Wetlands for Treating Ammonium-Nitrogen Contaminated Groundwater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic NH4+-N Contaminated Groundwater
2.2. Experimental Setup and Conditions
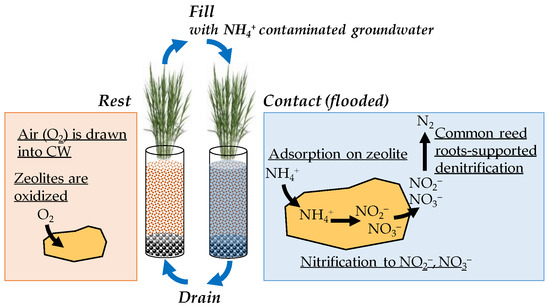
2.2.1. N Removal from NH4+-N Contaminated Groundwater by Tidal Flow CWs and Continuous Flow CWs
2.2.2. Evaluation of Vegetation Effects on N Removal Ability in the Tidal Flow CW
2.2.3. Denitrification Experiments Using Common Reed Roots from Tidal Flow CWs
2.3. Analysis of Samples
2.4. Microbial Community Analyses
2.5. Statistical Analysis
3. Results
3.1. N Removal from NH4+-N Contaminated Groundwater by Tidal Flow and Continuous Flow CWs
3.2. Evaluation of Vegetation Effects on N Removal Ability in the Tidal Flow CWs
3.3. Denitrification Experiments Using Common Reed Roots from Tidal Flow CWs
3.4. Characterization of Microbial Communities in Tidal Flow CWs with and without Vegetation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, M.; Cross, K.; Paden, M.; Laban, P. Spring: Managing Groundwater Sustainably; Smith, M., Cross, K., Paden, M., Laban, P., Eds.; IUCN: Gland, Switzerland, 2016. [Google Scholar] [CrossRef] [Green Version]
- Patterson, B.M.; Grassi, M.E.; Davis, G.B.; Robertson, B.S.; McKinley, A.J. Use of polymer mats in series for sequential reactive barrier remediation of ammonium-contaminated groundwater: Laboratory column evaluation. Environ. Sci. Technol. 2002, 36, 3439–3445. [Google Scholar] [CrossRef] [PubMed]
- Lindenbaum, J. Identification of Sources of Ammonium in Groundwater Using Stable Nitrogen and Boron Isotopes in Nam Du, Hanoi. Master’s Thesis, Lund University, Lund, Sweden, 2012. [Google Scholar]
- Huang, G.; Liu, F.; Yang, Y.; Kong, X.; Li, S.; Zhang, Y.; Cao, D. Ammonium-nitrogen-contaminated groundwater remediation by a sequential three-zone permeable reactive barrier (multibarrier) with oxygen-releasing compound (ORC)/clinoptilolite/spongy iron: Column studies. Environ. Sci. Pollut. Res. 2015, 22, 3705–3714. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Ramteke, S.; Rajhans, K.P.; Sahu, P.K.; Chakradhari, S.; Patel, K.S.; Matini, L. Assessment of Groundwater Quality in Central India. J. Water Resour. Prot. 2016, 8, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Shakya, B.M.; Nakamura, T.; Kamei, T.; Shrestha, S.D.; Nishida, K. Seasonal groundwater quality status and nitrogen contamination in the shallow aquifer system of the Kathmandu Valley, Nepal. Water 2019, 11, 2184. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Liu, F.; Yang, Y.; Deng, W.; Li, S.; Huang, Y.; Kong, X. Removal of ammonium-nitrogen from groundwater using a fully passive permeable reactive barrier with oxygen-releasing compound and clinoptilolite. J. Environ. Manag. 2015, 154, 1–7. [Google Scholar] [CrossRef]
- Li, S.; Huang, G.; Kong, X.; Yang, Y.; Liu, F.; Hou, G.; Chen, H. Ammonium removal from groundwater using a zeolite permeable reactive barrier: A pilot-scale demonstration. Water Sci. Technol. 2014, 70, 1540–1547. [Google Scholar] [CrossRef]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef]
- Brix, H. How “green” are aquaculture, constructed wetlands and conventional wastewater treatment systems? Water Sci. Technol. 1999, 40, 45–50. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Vymazal, J.; Kropfelova, L. Wastewater Treatment in Constructed Wetlands with Horizontal Sub-Surface Flow; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Abdelhakeem, S.G.; Aboulroos, S.A.; Kamel, M.M. Performance of a vertical subsurface flow constructed wetland under different operational conditions. J. Adv. Res. 2016, 7, 803–814. [Google Scholar] [CrossRef] [Green Version]
- Gikas, G.D.; Tsihrintzis, V.A. A small-size vertical flow constructed wetland for on-site treatment of household wastewater. Ecol. Eng. 2012, 44, 337–343. [Google Scholar] [CrossRef]
- Yousefi, Z.; Mohseni-Bandpei, A. Nitrogen and phosphorus removal from wastewater by subsurface wetlands planted with Iris pseudacorus. Ecol. Eng. 2010, 36, 777–782. [Google Scholar] [CrossRef]
- Lin, Y.F.; Jing, S.R.; Lee, D.Y.; Chang, Y.F.; Shih, K.C. Nitrate removal from groundwater using constructed wetlands under various hydraulic loading rates. Bioresour. Technol. 2008, 99, 7504–7513. [Google Scholar] [CrossRef] [PubMed]
- Braeckevelt, M.; Reiche, N.; Trapp, S.; Wiessner, A.; Paschke, H.; Kuschk, P.; Kaestner, M. Chlorobenzene removal efficiencies and removal processes in a pilot-scale constructed wetland treating contaminated groundwater. Ecol. Eng. 2011, 37, 903–913. [Google Scholar] [CrossRef]
- Amon, J.P.; Agrawal, A.; Shelley, M.L.; Opperman, B.C.; Enright, M.P.; Clemmer, N.D.; Slusser, T.; Lach, J.; Sobolewski, T.; Gruner, W.; et al. Development of a wetland constructed for the treatment of groundwater contaminated by chlorinated ethenes. Ecol. Eng. 2007, 30, 51–66. [Google Scholar] [CrossRef]
- Bedessem, M.E.; Ferro, A.M.; Hiegel, T. Pilot-scale constructed wetlands for petroleum-contaminated groundwater. Water Environ. Res. 2007, 79, 581–586. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Seeger, E.; Dorer, C.; Sinke, A.; Thullner, M. Performance of pilot-scale horizontal subsurface flow constructed wetlands treating groundwater contaminated with phenols and petroleum derivatives. Ecol. Eng. 2016, 95, 514–526. [Google Scholar] [CrossRef]
- Seeger, E.M.; Kuschk, P.; Fazekas, H.; Grathwohl, P.; Kaestner, M. Bioremediation of benzene-, MTBE- and ammonia-contaminated groundwater with pilot-scale constructed wetlands. Environ. Pollut. 2011, 159, 3769–3776. [Google Scholar] [CrossRef]
- Lee, C.; Fletcher, T.D.; Sun, G. Nitrogen removal in constructed wetland systems. Eng. Life Sci. 2009, 9, 11–22. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, C.; Liu, G.; Chen, Y.; Zhou, Q. Enhanced nitrogen removal reliability and efficiency in integrated constructed wetland microcosms using zeolite. Front. Environ. Sci. Eng. 2012, 6, 140–147. [Google Scholar] [CrossRef]
- Reddy, K.R.; Patrick, W.H. Nitrogen transformations and loss in flooded soils and sediments. Crit. Rev. Environ. Sci. Technol. 1984, 13, 273–309. [Google Scholar] [CrossRef]
- Vymazal, J. Horizontal sub-surface flow and hybrid constructed wetlands systems for wastewater treatment. Ecol. Eng. 2005, 25, 478–490. [Google Scholar] [CrossRef]
- Sun, G.J.; Zhao, Y.; Allen, S.; Cooper, D. Generating “tide” in pilot-scale constructed wetlands to enhance agricultural wastewater treatment. Eng. Life Sci. 2006, 6, 560–565. [Google Scholar] [CrossRef]
- Austin, D.; Lohan, E.; Verson, E. Nitrification and denitrification in a tidal vertical flow wetland pilot. In Water Environment Technical Conference; WEF: Los Angeles, CA, USA, 2003; pp. 333–357. [Google Scholar] [CrossRef] [Green Version]
- Austin, D. Influence of cation exchange capacity (CEC) in a tidal flow, flood and drain wastewater treatment wetland. Ecol. Eng. 2006, 28, 35–43. [Google Scholar] [CrossRef]
- Bruch, I.; Fritsche, J.; Bänninger, D.; Alewell, U.; Sendelov, M.; Hürlimann, H.; Hasselbach, R.; Alewell, C. Improving the treatment efficiency of constructed wetlands with zeolite-containing filter sands. Bioresour. Technol. 2011, 102, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Shuib, N.; Baskaran, K.; Jegatheesan, V. Evaluating the performance of horizontal subsurface flow constructed wetlands using natural zeolite (escott ). Int. J. Environ. Sci. Dev. 2011, 2, 311–315. [Google Scholar]
- Saeed, T.; Sun, G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: Dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manag. 2012, 112, 429–448. [Google Scholar] [CrossRef]
- Khanitchaidecha, W.; Shakya, M.; Nakano, Y.; Tanaka, Y.; Kazama, F. Development of an attached growth reactor for NH4-N removal at a drinking water supply system in Kathmandu Valley, Nepal. J. Environ. Sci. Health Part A 2012, 47, 734–743. [Google Scholar] [CrossRef]
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; Clesceri, L.S., Greenberg, A.E., Eaton, A.D., Eds.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 1998. [Google Scholar]
- Bru, D.; Martin-Laurent, F.; Philippot, L. Quantification of the detrimental effect of a single primer-template mismatch by real-time PCR using the 16S rRNA gene as an example. Appl. Environ. Microbiol. 2008, 74, 1660–1663. [Google Scholar] [CrossRef] [Green Version]
- Dionisi, H.M.; Layton, A.C.; Harms, G.; Gregory, I.R.; Robinson, K.G.; Sayler, G.S. Quantification of Nitrosomonas oligotropha-like ammonia-oxidizing bacteria and Nitrospira spp. from full-scale wastewater treatment plants by competitive PCR. Appl. Environ. Microbiol. 2002, 68, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Poly, F.; Wertz, S.; Brothier, E.; Degrange, V. First exploration of Nitrobacter diversity in soils by a PCR cloning-sequencing approach targeting functional gene nxrA. FEMS Microbiol. Ecol. 2008, 63, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Gutierrez, J.C.; Henry, S.; Hallet, S.; Martin-Laurent, F.; Catroux, G.; Philippot, L. Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. J. Microbiol. Methods 2004, 57, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Fields, M.W.; Wu, L.; Zu, Y.; Tiedje, J.M.; Zhou, J. Molecular diversity and characterization of nitrite reductase gene fragments (nirK and nirS) from nitrate- and uranium-contaminated groundwater. Environ. Microbiol. 2003, 5, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Throback, I.N.; Enwall, K.; Jarvis, A.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Scala, D.J.; Kerkhof, L.J. Nitrous oxide reductase (nosZ) gene-specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiol. Lett. 1998, 162, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sickle-A Windowed Adaptive Trimming Tool for FASTQ Files Using Quality. Available online: https://github.com/najoshi/sickle (accessed on 2 March 2020).
- Fastx-Toolkit. Available online: http://hannonlab.cshl.edu/fastx_toolkit/download.html (accessed on 2 March 2020).
- Flash. Available online: https://sourceforge.net/projects/flashpage/files/ (accessed on 2 March 2020).
- Usearch. Available online: https://www.drive5.com/usearch/ (accessed on 2 March 2020).
- Lin, Y.F.; Jing, S.R.; Wang, T.W.; Lee, D.Y. Effects of macrophytes and external carbon sources on nitrate removal from groundwater in constructed wetlands. Environ. Pollut. 2002, 119, 413–420. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Samadi, M.T.; Ehsani, H.R. Investigation of clinoptilolite natural zeolite regeneration by air stripping followed by ion exchange for removal of ammonium from aqueous solutions. Iran. J. Environ. Health Sci. Eng. 2009, 6, 167–172. [Google Scholar]
- Deng, Q.; Elbeshbishy, E.; Lee, H.S. Simultaneous regeneration of exhausted zeolite and nitrogen recovery using an air stripping method at alkaline pH. Water Qual. Res. J. Can. 2016, 51, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Li, M.; Zhang, Z.; Feng, C.; Bai, W.; Sugiura, N. Electrochemical regeneration of zeolites and the removal of ammonia. J. Hazard. Mater. 2009, 169, 746–750. [Google Scholar] [CrossRef]
- Lahav, O.; Green, M. Bioregenerated ion-exchange process: The effect of the biofilm on the ion-exchange capacity and kinetics. Water SA 2000, 26, 51–57. [Google Scholar]
- Rahmani, A.R.; Mahvi, A.H. Use of Ion Exchange for Removal of Ammonium: A Biological Regeneration of Zeolite. Glob. NEST J. 2006, 8, 146–150. [Google Scholar] [CrossRef]
- Sun, G.; Zhao, Y.; Allen, S. Enhanced removal of organic matter and ammoniacal-nitrogen in a column experiment of tidal flow constructed wetland system. J. Biotechnol. 2005, 115, 189–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Zhang, D.; Austin, D.; Dong, R.; Pang, C. Evaluation of a lab-scale tidal flow constructed wetland performance: Oxygen transfer capacity, organic matter and ammonium removal. Ecol. Eng. 2011, 37, 1789–1795. [Google Scholar] [CrossRef]
- Li, C.; Wu, S.; Dong, R. Dynamics of organic matter, nitrogen and phosphorus removal and their interactions in a tidal operated constructed wetland. J. Environ. Manag. 2015, 151, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wu, S.; Zhang, T.; Mazur, R.; Pang, C.; Dong, R. Dynamics of nitrogen transformation depending on different operational strategies in laboratory-scale tidal flow constructed wetlands. Sci. Total Environ. 2014, 487, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Z.; Li, F.; Fan, J.; Zhang, J.; Li, F.; Hu, H. Effect of oxygen supply strategy on nitrogen removal of biochar-based vertical subsurface flow constructed wetland: Intermittent aeration and tidal flow. Chemosphere 2019, 223, 366–374. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Yang, Y.; Fan, X.; Tan, X.; Yin, W.; Liu, Y.; Zhou, Z. Double-layer substrate of shale ceramsite and active alumina tidal flow constructed wetland enhanced nitrogen removal from decentralized domestic sewage. Sci. Total Environ. 2020, 703, 135629. [Google Scholar] [CrossRef]
- Münch, C.; Kuschk, P.; Röske, I. Root stimulated nitrogen removal: Only a local effect or important for water treatment? Water Sci. Technol. 2005, 51, 185–192. [Google Scholar] [CrossRef]
- Zhu, T.; Sikora, F.J. Ammonium and nitrate removal in vegetated and unvegetated gravel bed microcosm wetlands. Water Sci. Technol. 1995, 32, 219–228. [Google Scholar] [CrossRef]
- Hu, Y.; He, F.; Ma, L.; Zhang, Y.; Wu, Z. Microbial nitrogen removal pathways in integrated vertical-flow constructed wetland systems. Bioresour. Technol. 2016, 207, 339–345. [Google Scholar] [CrossRef]
- Toyama, T.; Nishimura, Y.; Ogata, Y.; Sei, K.; Mori, K.; Ike, M. Effects of planting Phragmites australis on nitrogen removal, microbial nitrogen cycling, and abundance of ammonia-oxidizing and denitrifying microorganisms in sediments. Environ. Technol. 2015, 37, 478–485. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, H.; Ngo, H.H.; Guo, W.; Zhang, J.; Liu, C.; Liang, S.; Hu, Z.; Yang, Z.; Zhao, C. Microbial abundance and community in subsurface flow constructed wetland microcosms: Role of plant presence. Environ. Sci. Pollut. Res. 2016, 23, 4036–4045. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Dong, J.; Shen, Z.; Mou, R.; Zhou, Y.; Chen, X.; Fu, X.; Yang, C. Nitrogen removal of anaerobically digested swine wastewater by pilot-scale tidal flow constructed wetland based on in-situ biological regeneration of zeolite. Chemosphere 2019, 217, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, Y.; Tang, Z.; Huang, J.; Zhou, Q.; Vymazal, J. Effects of plant biomass on bacterial community structure in constructed wetlands used for tertiary wastewater treatment. Ecol. Eng. 2015, 84, 38–45. [Google Scholar] [CrossRef]
- Rich, J.J.; Heichen, R.S.; Bottomley, P.J.; Cromack, K.; Myrold, D.D. Community composition and functioning of denitrifying bacteria from adjacent meadow and forest soils. Appl. Environ. Microbiol. 2003, 69, 5974–5982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Yang, Y.; Li, X.; Zhou, Z.; Liu, C.; Liu, Y.; Yin, W.; Fan, X. Intensified nitrogen removal by heterotrophic nitrification aerobic denitrification bacteria in two pilot-scale tidal flow constructed wetlands: Influence of influent C/N ratios and tidal strategies. Bioresour. Technol. 2020, 302, 122803. [Google Scholar] [CrossRef]
- Chen, Z.; Vymazal, J.; Kuschk, P. Effects of tidal operation on pilot-scale horizontal subsurface flow constructed wetland treating sulfate rich wastewater contaminated by chlorinated hydrocarbons. Environ. Sci. Pollut. Res. 2017, 24, 1042–1050. [Google Scholar] [CrossRef]







| Target Gene | Abundance (copies g−1 of zeolite) | |
|---|---|---|
| with Vegetation | without Vegetation | |
| Bacterial 16S rRNA | (6.6 ± 0.8) × 1010 | (4.0 ± 0.4) × 108 |
| AOB-amoA | (2.6 ± 0.9) × 108 | (2.3 ± 0.3) × 106 |
| nxrA | (7.1 ± 2.6) × 104 | (7.4 ± 1.2) × 102 |
| narG | (8.6 ± 3.6) × 108 | (5.5 ± 0.5) × 106 |
| nirK | (1.4 ± 0.6) × 108 | (1.1 ± 0.4) × 106 |
| nirS | (3.1 ± 1.1) × 108 | (1.0 ± 0.6) × 106 |
| nosZ | (1.9 ± 0.1) × 108 | (5.8 ± 0.4) × 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharjan, A.K.; Mori, K.; Toyama, T. Nitrogen Removal Ability and Characteristics of the Laboratory-Scale Tidal Flow Constructed Wetlands for Treating Ammonium-Nitrogen Contaminated Groundwater. Water 2020, 12, 1326. https://doi.org/10.3390/w12051326
Maharjan AK, Mori K, Toyama T. Nitrogen Removal Ability and Characteristics of the Laboratory-Scale Tidal Flow Constructed Wetlands for Treating Ammonium-Nitrogen Contaminated Groundwater. Water. 2020; 12(5):1326. https://doi.org/10.3390/w12051326
Chicago/Turabian StyleMaharjan, Amit Kumar, Kazuhiro Mori, and Tadashi Toyama. 2020. "Nitrogen Removal Ability and Characteristics of the Laboratory-Scale Tidal Flow Constructed Wetlands for Treating Ammonium-Nitrogen Contaminated Groundwater" Water 12, no. 5: 1326. https://doi.org/10.3390/w12051326