Nutrient Recovery from Anaerobically Treated Blackwater and Improving Its Effluent Quality through Microalgae Biomass Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Media and Strain
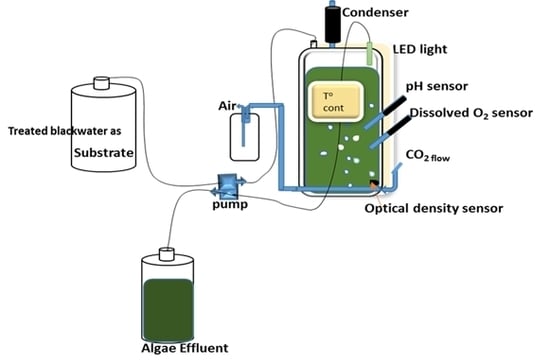
2.2. Photobioreactor (PBR) Set-Up and Culture Conditions
2.3. Nutrient Analysis and Algae Growth Determination
2.4. Statistical Analysis
3. Results
3.1. Biomass Productivity and Nutrient Removal with a Defined Medium
3.2. Biomass Productivity and Nutrient Removal with Treated Blackwater as a Substrate
3.3. Effect of NO2-N on Chlorella Sorokiniana
3.4. Effects of Reducing Substrate Retention Time, Stopping Stirring and Removing CaCl2 as a Supplement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kujawa-Roeleveld, K.; Zeeman, G. Anaerobic treatment in decentralized and source-separation-based. Rev. Environ. Sci. Bio/Technol 2006, 5, 115–139. [Google Scholar] [CrossRef]
- De Graaff, M.S.; Temmink, H.; Zeeman, G.; Buisman, C.J. Anaerobic treatment of concentrated black water in a uasb reactor at a short hrt. Water 2010, 2, 101–119. [Google Scholar] [CrossRef]
- De Graaff, M.H.; Temmink, G.; Zeeman, C. Buisman. Energy and phosphorus recovery from black water. Water Sci. Technol. 2011, 63, 2759–2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moges, M.E.; Todt, D.; Janka, E.; Heistad, A.; Bakke, R. Sludge blanket anaerobic baffled reactor for source-separated blackwater treatment. Water Sci. Technol 2018, 78, 1249–1259. [Google Scholar] [CrossRef] [Green Version]
- Zeeman, G.; Kujawa, K.; Mes, T.D.; Hernandez, L.; Graaff, M.D.; Abu-Ghunmi, L.; Mels, A.; Meulman, B.; Temmink, H.; Buisman, C.; et al. Anaerobic treatment as a core technology for energy, nutrients and water recovery from source-separated domestic waste (water). Water Sci. Technol 2008, 57, 1207–1212. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, W.; Drosg, B. Assessment of the state of the art of technologies for the processing of digestate residue from anaerobic digesters. Water Sci. Technol. 2013, 67, 1984–1993. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, Y.; Chen, P.; Min, M.; Chen, Y.; Zhu, J.; Ruan, R. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green Microalgae chlorella sp. Bioresour. Technol. 2010, 101, 2623–2628. [Google Scholar] [CrossRef]
- Vázquez-Padín, J.R.; Figueroa, M.; Fernández, I.; Mosquera-Corral, A.; Campos, J.L.; Mendez, R. Post-treatment of effluents from anaerobic digesters by the anammox process. Water Sci. Technol. 2009, 60, 1135–1143. [Google Scholar] [CrossRef]
- Ruiz, G.; Jeison, D.; Rubilar, O.; Ciudad, G.; Chamy, R. Nitrification–denitrification via nitrite accumulation for nitrogen removal from wastewaters. Bioresour. Technol. 2006, 97, 330–335. [Google Scholar] [CrossRef]
- Gao, D.; Peng, Y.; Li, B.; Liang, H. Shortcut nitrification–denitrification by real-time control strategies. Bioresour. Technol 2009, 100, 2298–2300. [Google Scholar] [CrossRef]
- Gao, D.; Peng, Y.; Wu, W.M. Kinetic model for biological nitrogen removal using shortcut nitrification-denitrification process in sequencing batch reactor. Environ. Sci. Technol. 2010, 44, 5015–5021. [Google Scholar] [CrossRef] [PubMed]
- Helmer, C.; Kunst, S. Simultaneous nitrification/denitrification in an aerobic biofilm system. Water Sci. Technol. 1998, 37, 183–187. [Google Scholar] [CrossRef]
- Yilmaz, G.; Lemaire, R.; Keller, J.; Yuan, Z. Simultaneous nitrification, denitrification, and phosphorus removal from nutrient-rich industrial wastewater using granular sludge. Biotechnol. Bioeng. 2008, 100, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Virdis, B.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Simultaneous nitrification, denitrification and carbon removal in microbial fuel cells. Water Res. 2010, 44, 2970–2980. [Google Scholar] [CrossRef]
- Fux, C.; Boehler, M.; Huber, P.; Brunner, I.; Siegrist, H. Biological treatment of ammonium-rich wastewater by partial nitritation and subsequent anaerobic ammonium oxidation (anammox) in a pilot plant. J. Biotechnol. 2002, 99, 295–306. [Google Scholar] [CrossRef]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C. Full-scale partial nitritation/anammox experiences–an application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef]
- Sun, S.-P.; Nàcher, C.P.i.; Merkey, B.; Zhou, Q.; Xia, S.Q.; Yang, D.H.; Sun, J.H.; Smets, B.F. Effective biological nitrogen removal treatment processes for domestic wastewaters with low c/n ratios: A review. Environ. Eng. Sci. 2010, 27, 111–126. [Google Scholar] [CrossRef] [Green Version]
- Kebede-Westhead, E.; Pizarro, C.; Mulbry, W.W. Treatment of dairy manure effluent using freshwater algae: Elemental composition of algal biomass at different manure loading rates. J. Agric. Food. Chem. 2004, 52, 7293–7296. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Claxton, R.; Das, K.C. Biomass and bioenergy production potential of microalgae consortium in open and closed bioreactors using untreated carpet industry effluent as growth medium. Bioresour. Technol. 2010, 101, 6751–6760. [Google Scholar] [CrossRef]
- IPCC. Climate Change—The Physical Science Basis: Working Group 1 Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; p. 212. [Google Scholar]
- Olguín, E.J. Phycoremediation: Key issues for cost-effective nutrient removal processes. Biotechnol. Adv. 2003, 22, 81–91. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.X.; Li, L.; Martinez, B.; Chen, P.; Ruan, R. Culture of microalgae chlamydomonas reinhardtii in wastewater for biomass feedstock production. Appl. Biochem. Biotechnol. 2010, 160, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae chlorella sp. In different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Tuantet, K.; Temmink, H.; Zeeman, G.; Janssen, M.; Wijffels, R.H.; Buisman, C.J. Nutrient removal and microalgal biomass production on urine in a short light-path photobioreactor. Water Res. 2014, 55, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos Fernandes, T.; Shrestha, R.; Sui, Y.; Papini, G.; Zeeman, G.; Vet, L.E.; Wijffels, R.H.; Lamers, P. Closing domestic nutrient cycles using microalgae. Environ. Sci. Technol. 2015, 49, 12450–12456. [Google Scholar] [CrossRef]
- Singh, M.; Reynolds, D.L.; Das, K.C. Microalgal system for treatment of effluent from poultry litter anaerobic digestion. Bioresour. Technol. 2011, 102, 10841–10848. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Chen, P.; Ruan, R. Semi-continuous cultivation of chlorella vulgaris for treating undigested and digested dairy manures. Appl. Biochem. Biotechnol. 2010, 162, 2324–2332. [Google Scholar] [CrossRef]
- Eshetu Moges, M.; Todt, D.; Heistad, A. Treatment of source-separated blackwater: A decentralized strategy for nutrient recovery towards a circular economy. Water 2018, 10, 463. [Google Scholar] [CrossRef] [Green Version]
- Hutner, S.H.; Provasoli, L.; Schatz, A.; Haskins, C.P. Some approaches to the study of the role of metals in the metabolism of microorganisms. Proc. Am. Philos. Soc. 1950, 94, 152–170. [Google Scholar]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221, 230A. [Google Scholar]
- NORCCA. Available online: https://niva-cca.no/shop/trebouxiophyceae/chlorella/niva-chl-176 (accessed on 21 February 2020).
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, R.; Hotta, M.; Masuda, Y.; Chihara, M.; Karube, I. Antioxidants from carbon dioxide fixing chlorella sorokiniana. J. Appl. Phycol. 2000, 12, 263–267. [Google Scholar] [CrossRef]
- Skjånes, K.; Andersen, U.; Heidorn, T.; Borgvang, S.A. Design and construction of a photobioreactor for hydrogen production, including status in the field. J. Appl. Phycol. 2016, 28, 2205–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Camejo, J.; Barat, R.; Pachés, M.; Murgui, M.; Seco, A.; Ferrer, J. Wastewater nutrient removal in a mixed microalgae–bacteria culture: Effect of light and temperature on the microalgae–bacteria competition. Environ. Technol. 2018, 39, 503–515. [Google Scholar] [CrossRef]
- Cuaresma, M.; Janssen, M.; Vílchez, C.; Wijffels, R.H. Horizontal or vertical photobioreactors? How to improve microalgae photosynthetic efficiency. Bioresour. Technol. 2011, 102, 5129–5137. [Google Scholar] [CrossRef]
- Tuantet, K.; Janssen, M.; Temmink, H.; Zeeman, G.; Wijffels, R.H.; Buisman, C.J. Microalgae growth on concentrated human urine. J. Appl. Phycol. 2014, 287–297. [Google Scholar] [CrossRef]
- Fernandes, T.V.; Suárez-Muñoz, M.; Trebuch, L.M.; Verbraak, P.J.; Van de Waal, D.B. Toward an ecologically optimized n: P recovery from wastewater by microalgae. Front. Microbiol. 2017, 8, 1742. [Google Scholar] [CrossRef] [Green Version]
- Maguer, J.F.; L’Helguen, S.; Madec, C.; Labry, C.; Le Corre, P. Nitrogen uptake and assimilation kinetics in alexandrium minutum (dynophyceae): Effect of n-limited growth rate on nitrate and ammonium interactions. Eur. J. Phycol. 2007, 43, 295–303. [Google Scholar] [CrossRef]
- Hii, Y.S.; SOO, C.L.; Chuah, T.S.; Mohd-Azmi, A.; Abol-Munafi, A.B. Interactive effect of ammonia and nitrogen on the nitrogen uptake by Nannochloropsis sp. J. Sustain. Sci. Manage 2011, 6, 60–68. [Google Scholar]
- Gonzalez-Camejo, J.; Barat, R.; Ruano, M.V.; Seco, A.; Ferrer, J. Outdoor flat-panel membrane photobioreactor to treat the effluent of an anaerobic membrane bioreactor. Influence of operating, design, and environmental conditions. Water Sci. Technol. 2018, 78, 195–206. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Podevin, M.; De Francisci, D.; Holdt, S.L.; Angelidaki, I. Effect of nitrogen source and acclimatization on specific growth rates of microalgae determined by a high-throughput in vivo microplate autofluorescence method. J. Appl. Phycol. 2015, 27, 1415–1423. [Google Scholar] [CrossRef] [Green Version]










| Nutrient | Anaerobically Digested Blackwater | After Filtration and UV Treatment | 10% ** TreatedBW | Defined Medium |
|---|---|---|---|---|
| Ntot mg L−1 | 1140–2360 | 1100–1980 | 110–198 | 247 |
| NH4-N mg L−1 | 580–1390 | 580–1200 | 58–120 | ND |
| NO3-N mg L−1 | 0–4.05 | 6–93.6 | 0.6–9.36 | 247 |
| NO2-N mg L−1 | 0–0.6 | 0.2–130 | 0.02–13 | ND |
| Ptot mg L−1 | 100–140 | 76–92.4 | 7.6–9.24 | 29.2 |
| PO4-P mg L−1 | 46–92.5 | 54–85.3 | 5.4–8.53 | 29.2 |
| Mg mg L−1 * | 11.3 | 9.4 | 0.94 | 9.8 |
| K mg L−1 * | 165.0 | 185.0 | 18.5 | 59.3 |
| Ca mg L−1 * | 38.8 | 31.8 | 3.18 | 13.6 |
| Na mg L−1 * | 191.7 | 195 | 19.5 | 411 |
| Al µg L−1 * | 22.0 | 15.5 | 1.55 | 0.0 |
| Fe µg L−1 * | 143.3 | 40.7 | 4.07 | 1.0 |
| Cu µg L−1 * | 30.7 | 6.9 | 0.69 | 0.4 |
| Mn µg L−1 * | 37.5 | 6.0 | 0.60 | 1.4 |
| Ni µg L−1 * | 11.1 | 3.7 | 0.37 | 0.0 |
| Zn µg L−1 * | 62.0 | 30.5 | 3.05 | 5.0 |
| Co µg L−1 * | 0.43 | 0.17 | 0.017 | 0.4 |
| Turbidity NTU | 80–160 | 0.15–2 | ND | ND |
| E. coli MPN/100 mL | 10^5 | <1 | <1 | ND |
| Unit | Defined Medium | 10% Treated BW without Extra P | 10% Treated BW with Extra P | 20% Treated BW with Extra P | |
|---|---|---|---|---|---|
| Ntot | mg L−1 | 247 | 54–85 | 54–85 | 177–212 |
| Ptot | mg L−1 | 29.2 | 4.5–6 | 33–35.2 | 18.8–21.3 |
| N:P ratio | 8.7 | 12–14 | 3.5 | 10 | |
| Volumetric biomass productivity PV | g L−1 d−1 | 2.17 | 1.5 | 2.1 | 1.9 |
| Areal biomass productivity PA | g m−1 d−1 | 52.21 | 36.18 | 50.43 | 46.04 |
| N removal efficiency Nreff | % | 80–98 | 99.8 | 99.7 | 77.8 |
| P removal efficiency Preff | % | 63–83 | 99.2 | 86.1 | 99.5 |
| N removal rate Nr | mg N L−1d−1 | 291.1 ± 29.8 | 99.17 ± 0.3 | 110.46 ± 0.4 | 212.6 ± 23 |
| P removal rate Pr | mg P L−1d−1 | 29.5 ± 4.1 | 8.3 ± 04 | 42.7 ± 2.6 | 35.3 ± 0.6 |
| N removal yield on light YN/Ph | mg (mole photons)−1 | 55.9 ± 5.7 | 19.1 ± 0.3 | 20.9 ± 0.1 | 41.0 ± 4.1 |
| P removal yield on light YP/Ph | mg (mole photons)−1 | 5.7 ± 0.8 | 1.6 ± 0.01 | 8 ± 0.9 | 6.8 ± 0.1 |
| Biomass yield on light YX/Ph | mg (mole photons)−1 | 420 | 290 | 400 | 370 |
| Biomass yield on N YX/N | g g−1 | 7.9 ± 1.3 | 15.2 ± 0.2 | 18.9 ± 1.5 | 9 ± 1.1 |
| Biomass yield on P YX/P | g g−1 | 78.4 ± 9.0 | 187.9 ± 9.5 | 50.2 ± 4.5 | 54 ± 5.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moges, M.E.; Heistad, A.; Heidorn, T. Nutrient Recovery from Anaerobically Treated Blackwater and Improving Its Effluent Quality through Microalgae Biomass Production. Water 2020, 12, 592. https://doi.org/10.3390/w12020592
Moges ME, Heistad A, Heidorn T. Nutrient Recovery from Anaerobically Treated Blackwater and Improving Its Effluent Quality through Microalgae Biomass Production. Water. 2020; 12(2):592. https://doi.org/10.3390/w12020592
Chicago/Turabian StyleMoges, Melesse Eshetu, Arve Heistad, and Thorsten Heidorn. 2020. "Nutrient Recovery from Anaerobically Treated Blackwater and Improving Its Effluent Quality through Microalgae Biomass Production" Water 12, no. 2: 592. https://doi.org/10.3390/w12020592