Wastewater Treatment by Novel Polyamide/Polyethylenimine Nanofibers with Immobilized Laccase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
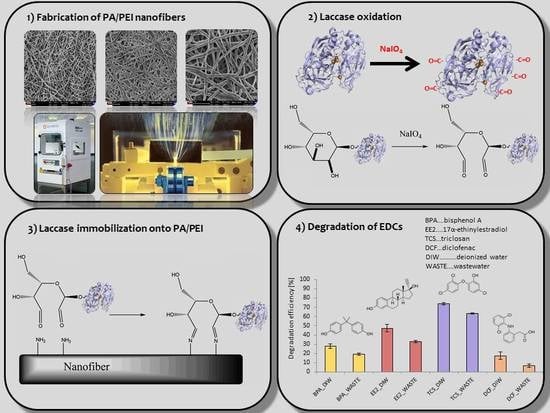
2.2. Preparation and Characterization of the Nanofiber Matrix
2.3. Characterization of Amino Groups
2.4. Measurement of Laccase Activity
2.5. Laccase Immobilization
2.6. Storage Stability and Reusability of Immobilized Laccase
2.7. Degradation of Endocrine-Disrupting Chemicals
3. Results
3.1. Nanofiber Characterization
Quantification of Amino Groups
3.2. Optimal Conditions for Laccase Immobilization
3.2.1. Effect of Buffer Concentration and pH on the Immobilization of Laccase onto PA/PEI Nanofibers
3.2.2. Effect of Concentration of NaIO4 and Oxidation Time on the Immobilization of Laccase onto PA/PEI Nanofibers
3.2.3. Effect of Immobilization Time on the Immobilization of Laccase onto PA/PEI Nanofibers
3.3. Summary of the Optimal Immobilization Process
3.4. Storage Stability and Reusability of Immobilized Laccase
3.5. EDCs Degradation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- López-Pacheco, I.Y.; Silva-Núñez, A.; Salinas-Salazar, C.; Arévalo-Gallegos, A.; Lizarazo-Holguin, L.A.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Anthropogenic contaminants of high concern: Existence in water resources and their adverse effects. Sci. Total Environ. 2019, 690, 1068–1088. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, V.L.; Binks, S.P.; Olson, M.J. Human health risk assessment from the presence of human pharmaceuticals in the aquatic environment. Regul. Toxicol. Pharmacol. 2009, 53, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Hybrid treatment systems for dye wastewater. Crit. Rev. Environ. Sci. Technol. 2007, 37, 315–377. [Google Scholar] [CrossRef]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef]
- Xu, R.; Tang, R.; Zhou, Q.; Li, F.; Zhang, B. Enhancement of catalytic activity of immobilized laccase for diclofenac biodegradation by carbon nanotubes. Chem. Eng. J. 2015, 262, 88–95. [Google Scholar] [CrossRef]
- Costa, J.B.; Lima, M.J.; Sampaio, M.J.; Neves, M.C.; Faria, J.L.; Morales-Torres, S.; Tavares, A.P.M.; Silva, C.G. Enhanced biocatalytic sustainability of laccase by immobilization on functionalized carbon nanotubes/polysulfone membranes. Chem. Eng. J. 2019, 355, 974–985. [Google Scholar] [CrossRef]
- Alver, E.; Metin, A.Ü. Chitosan based metal-chelated copolymer nanoparticles: Laccase immobilization and phenol degradation studies. Int. Biodeterior. Biodegrad. 2017, 125, 235–242. [Google Scholar] [CrossRef]
- Rotková, J.; Šuláková, R.; Korecká, L.; Zdražilová, P.; Jandová, M.; Lenfeld, J.; Horák, D.; Bílková, Z. Laccase immobilized on magnetic carriers for biotechnology applications. J. Magn. Magn. Mater. 2009, 321, 1335–1340. [Google Scholar] [CrossRef]
- Kadam, A.A.; Jang, J.; Jee, S.C.; Sung, J.-S.; Lee, D.S. Chitosan-functionalized supermagnetic halloysite nanotubes for covalent laccase immobilization. Carbohydr. Polym. 2018, 194, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zeng, Z.; Du, C.; Huang, D.; Zeng, G.; Xiao, R.; Lai, C.; Xu, P.; Zhang, C.; Wan, J.; et al. Immobilized laccase on bentonite-derived mesoporous materials for removal of tetracycline. Chemosphere 2019, 222, 865–871. [Google Scholar] [CrossRef]
- Zdarta, J.; Antecka, K.; Frankowski, R.; Zgoła-Grześkowiak, A.; Ehrlich, H.; Jesionowski, T. The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci. Total Environ. 2018, 615, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Kashefi, S.; Borghei, S.M.; Mahmoodi, N.M. Covalently immobilized laccase onto graphene oxide nanosheets: Preparation, characterization, and biodegradation of azo dyes in colored wastewater. J. Mol. Liq. 2019, 276, 153–162. [Google Scholar] [CrossRef]
- Maryšková, M.; Ardao, I.; García-González, C.A.; Martinová, L.; Rotková, J.; Ševců, A. Polyamide 6/chitosan nanofibers as support for the immobilization of Trametes versicolor laccase for the elimination of endocrine disrupting chemicals. Enzym. Microb. Technol. 2016, 89, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Maryskova, M.; Rysova, M.; Novotny, V.; Sevcu, A. Polyamide-Laccase Nanofiber Membrane for Degradation of Endocrine-Disrupting Bisphenol A, 17α-ethinylestradiol, and Triclosan. Polymers 2019, 11, 1560. [Google Scholar] [CrossRef] [Green Version]
- Arıca, M.Y.; Altıntas, B.; Bayramoğlu, G. Immobilization of laccase onto spacer-arm attached non-porous poly(GMA/EGDMA) beads: Application for textile dye degradation. Bioresour. Technol. 2009, 100, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, L.; Liao, Q.; Chen, X.; Qian, Z.; Shen, J.; Liang, J.; Yao, J. Functionalization of cellulose with hyperbranched polyethylenimine for selective dye adsorption and separation. Cellulose 2016, 23, 3785–3797. [Google Scholar] [CrossRef]
- An, G.S.; Choi, S.W.; Kim, T.G.; Shin, J.R.; Kim, Y.-I.; Choi, S.-C.; Jung, Y.-G. Amino-functionalization of colloidal alumina particles for enhancement of the infiltration behavior in a silica-based ceramic core. Ceram. Int. 2017, 43, 157–161. [Google Scholar] [CrossRef]
- Hartwig, A.; Mulder, M.; Smolders, C.A. Surface amination of poly(acrylonitrile). Adv. Colloid Interface Sci. 1994, 52, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Hassani, T.; Ba, S.; Cabana, H. Formation of enzyme polymer engineered structure for laccase and cross-linked laccase aggregates stabilization. Bioresour. Technol. 2013, 128, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Choi-Rhee, E.; Schulman, H.; Cronan, J.E. Promiscuous protein biotinylation by Escherichia coli biotin protein ligase. Protein Sci. 2004, 13, 3043–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Shannessy, D.J.; Hoffman, W.L. Site-directed immobilization of glycoproteins on hydrazide-containing solid supports. Biotechnol. Appl. Biochem. 1987, 9, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Bioconjugate Techniques, 3rd Edition. Available online: https://www.elsevier.com/books/bioconjugate-techniques/hermanson/978-0-12-382239-0 (accessed on 19 October 2018).
- Jolivalt, C.; Brenon, S.; Caminade, E.; Mougin, C.; Pontié, M. Immobilization of laccase from Trametes versicolor on a modified PVDF microfiltration membrane: characterization of the grafted support and application in removing a phenylurea pesticide in wastewater. J. Membr. Sci. 2000, 180, 103–113. [Google Scholar] [CrossRef]
- Li, G.; Nandgaonkar, A.G.; Wang, Q.; Zhang, J.; Krause, W.E.; Wei, Q.; Lucia, L.A. Laccase-immobilized bacterial cellulose/TiO2 functionalized composite membranes: Evaluation for photo- and bio-catalytic dye degradation. J. Membr. Sci. 2017, 525, 89–98. [Google Scholar] [CrossRef]
- Saravanakumar, T.; Park, H.-S.; Mo, A.-Y.; Choi, M.-S.; Kim, D.-H.; Park, S.-M. Detoxification of furanic and phenolic lignocellulose derived inhibitors of yeast using laccase immobilized on bacterial cellulosic nanofibers. J. Mol. Catal. B Enzym. 2016, 134, 196–205. [Google Scholar] [CrossRef]
- Kepp, K.P. Halide binding and inhibition of laccase copper clusters: the role of reorganization energy. Inorg. Chem. 2015, 54, 476–483. [Google Scholar] [CrossRef]
- Chapple, A.; Nguyen, L.N.; Hai, F.I.; Dosseto, A.; Rashid, M.H.-O.; Oh, S.; Price, W.E.; Nghiem, L.D. Impact of inorganic salts on degradation of bisphenol A and diclofenac by crude extracellular enzyme from Pleurotus ostreatus. Biocatal. Biotransform. 2019, 37, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Raseda, N.; Hong, S.; Kwon, O.Y.; Ryu, K. Kinetic evidence for the interactive inhibition of laccase from Trametes versicolor by pH and chloride. J. Microbiol. Biotechnol. 2014, 24, 1673–1678. [Google Scholar] [CrossRef] [Green Version]
- Nair, R.R.; Demarche, P.; Agathos, S.N. Formulation and characterization of an immobilized laccase biocatalyst and its application to eliminate organic micropollutants in wastewater. New Biotechnol. 2013, 30, 814–823. [Google Scholar] [CrossRef]
- Songulashvili, G.; Jimenéz-Tobón, G.A.; Jaspers, C.; Penninckx, M.J. Immobilized laccase of Cerrena unicolor for elimination of endocrine disruptor micropollutants. Fungal Biol. 2012, 116, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Hongyan, L.; Zexiong, Z.; Shiwei, X.; He, X.; Yinian, Z.; Haiyun, L.; Zhongsheng, Y. Study on transformation and degradation of bisphenol A by Trametes versicolor laccase and simulation of molecular docking. Chemosphere 2019, 224, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Olajuyigbe, F.M.; Adetuyi, O.Y.; Fatokun, C.O. Characterization of free and immobilized laccase from Cyberlindnera fabianii and application in degradation of bisphenol A. Int. J. Biol. Macromol. 2019, 125, 856–864. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, R.; García-García, A.; Orona-Navar, C.; Osma, J.F.; Nigam, K.D.P.; Ornelas-Soto, N. Biotransformation of emerging pollutants in groundwater by laccase from P. sanguineus CS43 immobilized onto titania nanoparticles. J. Environ. Chem. Eng. 2018, 6, 710–717. [Google Scholar] [CrossRef]






| Analyte | Conc. (mg/L) | Analyte | Conc. (mg/L) |
|---|---|---|---|
| Fluoride | 0.16 | Cr | <0.001 |
| Chloride | 213 | Cu | <0.001 |
| Nitrate | 9.5 | Fe | 0.003 |
| Nitrite | <0.05 | K | 74.8 |
| Sulfate | 9.5 | Mg | 21.3 |
| TOC | 6.2 | Mn | <0.001 |
| Ag | 0.0019 | Na | 119 |
| Al | 0.035 | Ni | 0.002 |
| Be | <0.001 | Pb | <0.01 |
| Ca | 127 | V | <0.01 |
| Co | <0.002 | Zn | 0.014 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maryšková, M.; Schaabová, M.; Tománková, H.; Novotný, V.; Rysová, M. Wastewater Treatment by Novel Polyamide/Polyethylenimine Nanofibers with Immobilized Laccase. Water 2020, 12, 588. https://doi.org/10.3390/w12020588
Maryšková M, Schaabová M, Tománková H, Novotný V, Rysová M. Wastewater Treatment by Novel Polyamide/Polyethylenimine Nanofibers with Immobilized Laccase. Water. 2020; 12(2):588. https://doi.org/10.3390/w12020588
Chicago/Turabian StyleMaryšková, Milena, Markéta Schaabová, Hana Tománková, Vít Novotný, and Miroslava Rysová. 2020. "Wastewater Treatment by Novel Polyamide/Polyethylenimine Nanofibers with Immobilized Laccase" Water 12, no. 2: 588. https://doi.org/10.3390/w12020588