Aquatic Macrophytes and Local Factors Drive Bacterial Community Distribution and Interactions in a Riparian Zone of Lake Taihu
Abstract
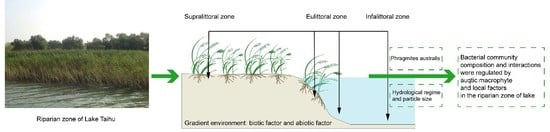
:1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling
2.2. Sample Chemical Properties
2.3. S rRNA Gene Pyrosequencing and Bioinformatics Analysis
2.4. Network Construction and Analysis
2.5. Statistical Analysis
3. Results
3.1. Local Factors in Sampling Zones
3.2. Bacterial Community Composition and Diversity in the Riparian Zone of Lake Taihu
3.3. Relationships between Bacterial Communities and Local Factors in the Riparian Zone of Lake Taihu
3.4. Co-Occurrence Bacterial Networks in the Riparian Zone of Lake Taihu
3.5. Relationships between Network Connectivity and Local Factors in the Riparian Zone of Lake Taihu
4. Discussion
4.1. Roots Enrich and Filter the Bacterial Community Composition in the Riparian Zone of Lake Taihu
4.2. Co-Occurrence Network Structures in the Riparian Zone of Lake Taihu
4.3. Links among Aquatic Macrophytes, Local Factors, and Bacterial Communities in the Riparian Zone of Lake Taihu
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McClain, M.E.; Boyer, E.W.; Dent, C.L.; Gergel, S.E.; Grimm, N.B.; Groffman, P.M.; Hart, S.C.; Harvey, J.W.; Johnston, C.A.; Mayorga, E.; et al. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 2003, 6, 301–312. [Google Scholar] [CrossRef]
- Srivastava, J.; Kalra, S.J.S.; Naraian, R. Environmental perspectives of Phragmites australis (Cav.) Trin. Ex. Steudel. Appl. Water Sci. 2014, 4, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Trias, R.; Ruiz-Rueda, O.; Garcia-Lledo, A.; Vilar-Sanz, A.; Lopez-Flores, R.; Quintana, X.D.; Hallin, S.; Baneras, L. Emergent macrophytes act selectively on ammonia-oxidizing bacteria and archaea. Appl. Environ. Microbiol. 2012, 78, 6352–6356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrangelo, L.; Bucci, A.; Maiuro, L.; Bulgarelli, D.; Naclerio, G. Unraveling the composition of the root-associated bacterial microbiota of Phragmites australis and Typha latifolia. Front. Microbiol. 2018, 9, 1650. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, J.K.; Chandra, H.; Kalra, S.J.S.; Mishra, P.; Khan, H.; Yadav, P. Plant–microbe interaction in aquatic system and their role in the management of water quality: A review. Appl. Water Sci. 2016, 7, 1079–1090. [Google Scholar] [CrossRef] [Green Version]
- Foulquier, A.; Volat, B.; Neyra, M.; Bornette, G.; Montuelle, B. Long-term impact of hydrological regime on structure and functions of microbial communities in riverine wetland sediments. FEMS Microbiol. Ecol. 2013, 85, 211–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peralta, A.L.; Matthews, J.W.; Kent, A.D. Habitat specialization along a wetland moisture gradient differs between ammonia-oxidizing and denitrifying microorganisms. Microb. Ecol. 2014, 68, 339–350. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, D.; Wu, J.; Chen, Q.; Long, C.; Li, Y.; Cheng, X. Anti-seasonal submergence dominates the structure and composition of prokaryotic communities in the riparian zone of the Three Gorges Reservoir, China. Sci. Total Environ. 2019, 663, 662–672. [Google Scholar] [CrossRef]
- Laurent, P.; Raaijmakers, J.M.; Philippe, L.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar]
- Haichar, F.e.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; He, X.; Zhang, S.; Liang, R.; Shen, J. Effects of root exudates on denitrifier gene abundance, community structure and activity in a micro-polluted constructed wetland. Sci. Total Environ. 2017, 598, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Behera, P.; Mahapatra, S.; Mohapatra, M.; Kim, J.Y.; Adhya, T.K.; Raina, V.; Suar, M.; Pattnaik, A.K.; Rastogi, G. Salinity and macrophyte drive the biogeography of the sedimentary bacterial communities in a brackish water tropical coastal lagoon. Sci. Total Environ. 2017, 595, 472–485. [Google Scholar] [CrossRef]
- Bowen, J.L.; Kearns, P.J.; Byrnes, J.E.K.; Wigginton, S.; Allen, W.J.; Greenwood, M.; Tran, K.; Yu, J.; Cronin, J.T.; Meyerson, L.A. Lineage overwhelms environmental conditions in determining rhizosphere bacterial community structure in a cosmopolitan invasive plant. Nat. Commun 2017, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Zhu, J.N.; Liu, Q.F.; Liu, Y.; Liu, M.; Liu, L.; Zhang, Q. Comparison of the diversity of root-associated bacteria in Phragmites australis and Typha angustifolia L. in artificial wetlands. World J. Microbiol. Biotechnol. 2013, 29, 1499–1508. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Yang, Y. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. mBio 2011, 2. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Tu, Q.; Zhi, X.; Relman, D.A. Functional Molecular Ecological Networks. mBio 2010, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Nuccio, E.E.; Shi, Z.J.; He, Z.; Zhou, J.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef] [Green Version]
- Fan, K.; Cardona, C.; Li, Y.; Shi, Y.; Xiang, X.; Shen, C.; Wang, H.; Gilbert, J.A.; Chu, H. Rhizosphere-associated bacterial network structure and spatial distribution differ significantly from bulk soil in wheat crop fields. Soil Biol. Biochem. 2017, 113, 275–284. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, S.; Li, R.; Zhang, J.; Liu, Y.; Lv, L.; Zhu, H.; Wu, W.; Li, W. Plant cultivars imprint the rhizosphere bacterial community composition and association networks. Soil Biol. Biochem. 2017, 109, 145–155. [Google Scholar] [CrossRef]
- Hamonts, K.; Trivedi, P.; Garg, A.; Janitz, C.; Grinyer, J.; Holford, P.; Botha, F.C.; Anderson, I.C.; Singh, B.K. Field study reveals core plant microbiota and relative importance of their drivers. Environ. Microbiol. 2018, 20, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D.; Kemen, E.M. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.F.; Zhang, T.; Yang, X.; Wang, S.; Yu, Y.; Dong, L.L.; Guo, Y.D.; Ma, Y.X.; Zang, J.Y. Diversity and composition of bacterial community in soils and lake sediments from an arctic lake area. Front. Microbiol. 2016, 7, 1170. [Google Scholar] [CrossRef] [Green Version]
- Deslippe, J.R.; Jamali, H.; Jha, N.; Saggar, S. Denitrifier community size, structure and activity along a gradient of pasture to riparian soils. Soil Biol. Biochem. 2014, 71, 48–60. [Google Scholar] [CrossRef]
- Ye, C.; Cheng, X.; Zhang, K.; Du, M.; Zhang, Q. Hydrologic pulsing affects denitrification rates and denitrifier communities in a revegetated riparian ecotone. Soil Biol. Biochem. 2017, 115, 137–147. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Z.; Xia, X.; Wang, F. A shallow lake remediation regime with Phragmites australis: Incorporating nutrient removal and water evapotranspiration. Water Res. 2012, 46, 5635–5644. [Google Scholar] [CrossRef] [PubMed]
- Luigimaria, B.; Giovanni, B.; Alessio, M.; Roberto, D.P.; Lorenzo, B. Rhizosphere effect and salinity competing to shape microbial communities in Phragmites australis (Cav.) Trin. ex-Steud. FEMS Microbiol. Lett. 2015, 359, 193–200. [Google Scholar]
- Packer, J.G.; Meyerson, L.A.; Skálová, H.; Pyšek, P.; Kueffer, C. Biological flora of the British Isles: Phragmites australis. J. Ecol. 2017, 105, 1123–1162. [Google Scholar] [CrossRef] [Green Version]
- Hallin, S.; Hellman, M.; Choudhury, M.I.; Ecke, F. Relative importance of plant uptake and plant associated denitrification for removal of nitrogen from mine drainage insub-arctic wetlands. Water Res. 2015, 85, 377–383. [Google Scholar] [CrossRef]
- Kowalski, K.P.; Bacon, C.; Bickford, W.; Braun, H.; Clay, K.; Leduc-Lapierre, M.; Lillard, E.; McCormick, M.K.; Nelson, E.; Torres, M.; et al. Advancing the science of microbial symbiosis to support invasive species management: A case study on Phragmites in the Great Lakes. Front. Microbiol. 2015, 6, 95. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, M.; Saunders, A.M.; Schramm, A. Effect of lake trophic status and rooted macrophytes on community composition and abundance of ammonia-oxidizing prokaryotes in freshwater sediments. Appl. Environ. Microbiol. 2009, 75, 3127–3136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, M.E. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Girvan, M.; Newman, M.E.J. Community structure in social and biological networks. Proc. Natl. Acad. Sci. USA 2002, 99, 7821–7826. [Google Scholar] [CrossRef] [Green Version]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The ecology of Acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef] [Green Version]
- Peiffer, J.A.; Aymé, S.; Omry, K.; Zhao, J.; Susannah Green, T.; Dangl, J.L.; Buckler, E.S.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.; Johnson, C.; Santos-Medellin, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [Green Version]
- Cardinale, M.; Grube, M.; Erlacher, A.; Quehenberger, J.; Berg, G. Bacterial networks and co-occurrence relationships in the lettuce root microbiota. Environ. Microbiol. 2015, 17, 239–252. [Google Scholar] [CrossRef]
- Sl, B.S.T.; Kuske, C.R. Wide distribution and diversity of members of the bacterial kingdom Acidobacterium in the environment. Appl. Environ. Microbiol. 1999, 65, 1731–1737. [Google Scholar]
- Lee, S.H.; Ka, J.O.; Cho, J.C. Members of the phylum Acidobacteria are dominant and metabolically active in rhizosphere soil. FEMS Microbiol. Lett. 2008, 285, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debruyn, J.M.; Nixon, L.T.; Fawaz, M.N.; Johnson, A.M.; Mark, R. Global biogeography and quantitative seasonal dynamics of Gemmatimonadetes in soil. Appl. Environ. Microbiol. 2011, 77, 6295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, N.; Geetanjali, G. Symbiotic nitrogen fixation in legume nodules: Process and signaling. A review. Agron. Sustain. Dev. 2007, 27, 59–68. [Google Scholar] [CrossRef]
- Ye, W.; Liu, X.; Lin, S.; Tan, J.; Pan, J.; Li, D.; Yang, H. The vertical distribution of bacterial and archaeal communities in the water and sediment of Lake Taihu. FEMS Microbiol. Ecol. 2009, 70, 107–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Krause, A.E.; Frank, K.A.; Mason, D.M.; Ulanowicz, R.E.; Taylor, W.W. Compartments revealed in food-web structure. Nature 2003, 426, 282–285. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive microbial metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Morohoshi, T.; Sato, N.; Iizumi, T.; Tanaka, A.; Ikeda, T. Identification and characterization of a novel N-acyl-homoserine lactonase gene in Sphingomonas ursincola isolated from industrial cooling water systems. J. Biosci. Bioeng. 2017, 123, 569–575. [Google Scholar] [CrossRef]
- DeAngelis, K.M.; Lindow, S.E.; Firestone, M.K. Bacterial quorum sensing and nitrogen cycling in rhizosphere soil. FEMS Microbiol. Ecol. 2008, 66, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Elasri, M.; Delorme, S.; Lemanceau, P.; Stewart, G.; Laue, B.; Glickmann, E.; Oger, P.M.; Dessaux, Y. Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soilborne Pseudomonas spp. Appl. Environ. Microbiol. 2001, 67, 1198–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, J.; Pierson, E.A.; Pierson, L.S.; Stacey, G.; Chatterjee, A. Quorum sensing in plant-associated bacteria. Curr. Opin. Plant. Biol. 2002, 5, 285–290. [Google Scholar] [CrossRef]
- Brown, S.D.; Utturkar, S.M.; Magnuson, T.S.; Ray, A.E.; Poole, F.L.; Lancaster, W.A.; Thorgersen, M.P.; Adams, M.W.; Elias, D.A. Complete genome sequence of Pelosinus sp. strain UFO1 assembled using single-molecule real-time DNA sequencing technology. Genome Announc. 2014, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, K.N.; Krumholz, L.R.; Gieg, L.M.; Parisi, V.A.; Suflita, J.M.; Allen, J.; Philp, R.P.; Elshahed, M.S. Biodegradation of low-molecular-weight alkanes under mesophilic, sulfate-reducing conditions: Metabolic intermediates and community patterns. FEMS Microbiol. Ecol. 2010, 72, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Frigaard, N.U.; Vogl, K.; Iino, T.; Ohkuma, M.; Overmann, J.; Bryant, D.A. Complete genome of Ignavibacterium album, a metabolically versatile, flagellated, facultative anaerobe from the phylum Chlorobi. Front. Microbiol. 2012, 3, 185. [Google Scholar] [CrossRef] [Green Version]
- Monard, C.; Gantner, S.; Bertilsson, S.; Hallin, S.; Stenlid, J. Habitat generalists and specialists in microbial communities across a terrestrial-freshwater gradient. Sci. Rep. 2016, 6, 37719. [Google Scholar] [CrossRef]
- Tanaka, N.; Yutani, K.; Aye, T.; Jinadasa, K.B.S.N. Effect of broken dead culms of Phragmites australis on radial oxygen loss in relation to radiation and temperature. Hydrobiologia 2007, 583, 165–172. [Google Scholar] [CrossRef]
- Mendes, L.W.; Kuramae, E.E.; Navarrete, A.A.; van Veen, J.A.; Tsai, S.M. Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J. 2014, 8, 1577–1587. [Google Scholar] [CrossRef]
- Ofek-Lalzar, M.; Sela, N.; Goldman-Voronov, M.; Green, S.J.; Hadar, Y.; Minz, D. Niche and host-associated functional signatures of the root surface microbiome. Nat. Commun. 2014, 5, 4950. [Google Scholar] [CrossRef]
- Yan, Y.; Kuramae, E.E.; De, H.M.; Klinkhamer, P.G.; van Veen, J.A. Functional traits dominate the diversity-related selection of bacterial communities in the rhizosphere. ISME J. 2016, 11, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Ruan, X.; Zhang, Y.; Li, R. Illumina sequencing-based analysis of sediment bacteria community in different trophic status freshwater lakes. Microbiologyopen 2017, 6, e00450. [Google Scholar] [CrossRef] [PubMed]
- Weise, L.; Ulrich, A.; Moreano, M.; Gessler, A.; Kayler, Z.E.; Steger, K.; Zeller, B.; Rudolph, K.; Knezevic-Jaric, J.; Premke, K. Water level changes affect carbon turnover and microbial community composition in lake sediments. FEMS Microbiol. Ecol. 2016, 92, fiw035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostendorp, W.; Dienst, M.; Schmieder, K. Disturbance and rehabilitation of lakeside Phragmites reeds following an extreme flood in Lake Constance (Germany). Hydrobiologia 2003, 50–509, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Santmire, J.A.; Leff, L.G. The influence of stream sediment particle size on bacterial abundance and community composition. Aquat. Ecol. 2006, 41, 153–160. [Google Scholar] [CrossRef]
- Jackson, C.R.; Weeks, A.Q. Influence of particle size on bacterial community structure in aquatic sediments as revealed by 16S rRNA gene sequence analysis. Appl. Environ. Microbiol. 2008, 74, 5237–5240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkacz, A.; Cheema, J.; Chandra, G.; Grant, A.; Poole, P.S. Stability and succession of the rhizosphere microbiota depends upon plant type and soil composition. ISME J. 2015, 9, 2349–2359. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Dohrmann, A.B.; Christensen, B.T.; Tebbe, C.C. Bacterial preferences for specific soil particle size fractions revealed by community analyses. Front. Microbiol. 2018, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Hemkemeyer, M.; Christensen, B.T.; Martens, R.; Tebbe, C.C. Soil particle size fractions harbour distinct microbial communities and differ in potential for microbial mineralisation of organic pollutants. Soil Biol. Biochem. 2015, 90, 255–265. [Google Scholar] [CrossRef]






| RZ | NZ | |||
|---|---|---|---|---|
| Bc | Wu | Bc | Wu | |
| All factors | 0.37(0.003) | 0.13(0.12) | 0.69(0.001) | 0.56(0.001) |
| NH4+ | −0.062(0.64) | −0.068(0.62) | 0.67(0.001) | 0.5(0.001) |
| NO3− | −0.044 (0.58) | 0.042(0.33) | 0.14 (0.2) | 0.22(0.084) |
| pH | 0.13(0.16) | −0.019(0.47) | 0.072(0.25) | 0.044(0.32) |
| TN | 0.31(0.079) | 0.24(0.13) | 0.49(0.001) | 0.56(0.001) |
| TC | 0.12(0.21) | 0.073(0.29) | 0.51 (0.001) | 0.54(0.002) |
| TC/TN | −0.026(0.55) | −0.013(0.45) | 0.063(0.26) | −0.012(0.46) |
| OM | 0.1(0.22) | 0.027(0.34) | 0.45(0.006) | 0.55(0.001) |
| SWC | 0.35(0.006) | 0.24(0.045) | 0.59(0.001) | 0.66(0.001) |
| DOC | −0.11 (0.76) | −0.11(0.75) | 0.24(0.029) | 0.36(0.011) |
| Clay | 0.042(0.37) | −0.049(0.55) | 0.66(0.001) | 0.056(0.001) |
| Silt | 0.35(0.007) | 0.078(0.21) | 0.1 (0.2) | 0.07(0.3) |
| Sand | 0.27(0.03) | −0.0067(0.47) | 0.5(0.003) | 0.38(0.008) |
| Networks | RZ | NZ |
|---|---|---|
| Empirical networks | ||
| No. of original OUT | 740 | 590 |
| Similarity threshold (st) | 0.86 | 0.89 |
| Network size | 377 | 322 |
| Total links | 556 | 909 |
| Positive links | 438 | 797 |
| Negative links | 118 | 112 |
| R2 of power law | 0.905 | 0.84 |
| Average path (GD) | 6.261 | 5.4 |
| Average degree (avgK) | 2.95 *** | 5.646 *** |
| Average clustering coefficient (avgCC) | 0.161 *** | 0.311 *** |
| Modularity and (the number of modules) | 0.802(17) | 0.676(10) |
| Random networks (SD) | ||
| Average path (GD) | 4.722(0.072) | 3.398(0.033) |
| Average clustering coefficient (avgCC) | 0.010(0.003) | 0.047(0.007) |
| Modularity (M) | 0.623(0.007) | 0.377(0.005) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
LYU, Y.; Huang, R.; Zeng, J.; Wu, Q.L. Aquatic Macrophytes and Local Factors Drive Bacterial Community Distribution and Interactions in a Riparian Zone of Lake Taihu. Water 2020, 12, 432. https://doi.org/10.3390/w12020432
LYU Y, Huang R, Zeng J, Wu QL. Aquatic Macrophytes and Local Factors Drive Bacterial Community Distribution and Interactions in a Riparian Zone of Lake Taihu. Water. 2020; 12(2):432. https://doi.org/10.3390/w12020432
Chicago/Turabian StyleLYU, Yuanjiao, Rui Huang, Jin Zeng, and Qinglong L. Wu. 2020. "Aquatic Macrophytes and Local Factors Drive Bacterial Community Distribution and Interactions in a Riparian Zone of Lake Taihu" Water 12, no. 2: 432. https://doi.org/10.3390/w12020432