Electrocoagulation: A Promising Method to Treat and Reuse Mineral Processing Wastewater with High COD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples, Reagents, and Analytical Methods
2.2. EC Experiments
2.3. Flotation Experiments
3. Results and Discussion
3.1. Effect of EC Parameters on COD Removal Rate of Mixed Wastewater
3.2. Effect of Additives on COD Removal Rate of Mixed Wastewater
3.3. Effect of EC Treatment on COD Removal Rate of Different Types of Wastewater
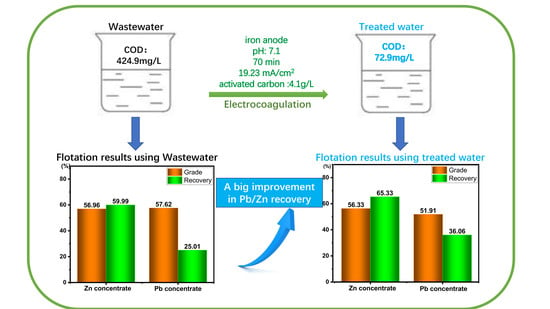
3.4. Effect of Water Type on the Grade and Recovery of Pb/Zn Sulfide Mineral Flotation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farrokhpay, S.; Zanin, M. An investigation into the effect of water quality on froth stability. Adv. Powder Technol. 2012, 23, 493–497. [Google Scholar] [CrossRef]
- Zhao, S.; Pan, J.Z. Overview on the Treatment Technology of Wastewater from Copper Mineral Processing (WCMP). Adv. Mater. Res. 2013, 750, 1369–1372. [Google Scholar]
- Sitorus, F.; Cilliers, J.J.; Brito-Parada, P.R. Multi-criteria decision making for the choice problem in mining and mineral processing: Applications and trends. Expert Syst. Appl. 2019, 121, 393–417. [Google Scholar] [CrossRef]
- Kobya, M.; Can, O.T.; Bayramoglu, M. Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J. Hazard. Mater. 2003, 99, 163–178. [Google Scholar] [CrossRef]
- Beck, E.C.; Giannini, A.P.; Ramirez, E.R. Electrocoagulation clarifies food wastewater. Food Technol. 1974, 22, 18–19. [Google Scholar]
- Bazrafshan, E. Application of electrocoagulation process for dairy wastewater treatment. J. Chem. 2012, 2013, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, Q. Experimental studies on pretreatment process of brackish water using electrocoagulation (EC) method. Desalination 1987, 66, 353–364. [Google Scholar] [CrossRef]
- Vik, E.A.; Carlson, D.A.; Eikum, A.S.; Gjessing, E.T. Electrocoagulation of potable water. Water Res. 1984, 18, 1355–1360. [Google Scholar] [CrossRef]
- Eryuruk, K.; Un, U.T.; Ogutveren, U.B. Electrochemical treatment of wastewaters from poultry slaughtering and processing by using iron electrodes. J. Clean. Prod. 2019, 121, 1089–1095. [Google Scholar] [CrossRef]
- Moreno-Casillas, H.A.; Cocke, D.L.; Gomes, J.A.G.; Morkovsky, P.; Parga, J.R.; Peterson, E. Electrocoagulation mechanism for COD removal. Sep. Purif. Technol. 2007, 56, 204–211. [Google Scholar] [CrossRef]
- Mollah, M.Y.; Morkovsky, P.; Gomes, J.A.; Kesmez, M.; Parga, J.; Cocke, D.L. Fundamentals, present and future perspectives of electrocoagulation. J. Hazard. Mater. 2004, 114, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.N. Ferric chloride and alum as single and dual coagulants. Acta Sci. Circumst. 1983, 75, 232–239. [Google Scholar] [CrossRef]
- Makarov, V.M.; Kalaeva, S.Z.; Zakharova, I.N.; Nevzorov, I.A.; Maltseva, M.S.; Shipilin, A.M.; Krzhizhanovskaya, M.G. Magnetic and X-ray studies of nanodispersed magnetite synthesized from chrome containing galvanic sludge. J. Nano- Electron. Phys. 2015, 7, 4–12. [Google Scholar]
- Wei, X.; Cao, J.; Holub, R.F.; Hopke, P.K.; Zhao, S. TEM study of geogas-transported nanoparticles from the Fankou lead–zinc deposit, Guangdong Province, South China. J. Geochem. Explor. 2013, 128, 124–135. [Google Scholar] [CrossRef]
- Yang, S.; Verdaguer-Casadevall, A.; Arnarson, L.; Silvioli, L.; Čolić, V.; Frydendal, R.; Rossmeisl, J.; Chorkendorff, I.; Stephens, I.E. Toward the decentralized electrochemical production of H2O2: a focus on the catalysis. Acs Catal. 2018, 8, 4064–4081. [Google Scholar] [CrossRef] [Green Version]
- Arslan-Alaton, İ.; Kabdaşlı, I.; Vardar, B.; Tünay, O. Electrocoagulation of simulated reactive dyebath effluent with aluminum and stainless steel electrodes. J. Hazard. Mater. 2009, 164, 1586–1594. [Google Scholar] [CrossRef]
- Ghalwa, A.; Nasser, M.; Farhat, N. Removal of abamectin pesticide by electrocoagulation process using stainless steel and iron electrodes. J. Env. Anal. Chem. 2015, 2, 134. [Google Scholar] [CrossRef] [Green Version]
- Garg, K.K.; Prasad, B. Treatment of toxic pollutants of purified terephthalic acid waste water: A review. Environ. Technol. Innov. 2017, 8, 191–217. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Yin, M.; Ma, X.; Lin, S. Removing heavy metal ions with continuous aluminum electrocoagulation: A study on back mixing and utilization rate of electro-generated Al ions. Chem. Eng. J. 2015, 267, 86–92. [Google Scholar] [CrossRef]
- Doǧan, D.; Türkdemir, H. Electrochemical oxidation of textile dye indigo. J. Chem. Technol. Biotechnol.: Int. Res. Process, Environ. Clean Technol. 2005, 80, 916–923. [Google Scholar]
- Zongo, I.; Maiga, A.H.; Wéthé, J.; Valentin, G.; Leclerc, J.P.; Paternotte, G.; Lapicque, F. Electrocoagulation for the treatment of textile wastewaters with Al or Fe electrodes: Compared variations of COD levels, turbidity and absorbance. J. Hazard. Mater. 2009, 169, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.K.; Barton, G.W.; Mitchell, C.A. The future for electrocoagulation as a localised water treatment technology. Chemosphere 2005, 59, 355–567. [Google Scholar] [CrossRef] [PubMed]
- Ghernaout, D.; Ghernaout, B. On the controversial effect of sodium sulphate as supporting electrolyte on electrocoagulation process: A review. Desalination Water Treat. 2011, 27, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chen, G.; Yue, P.L. Separation of pollutants from restaurant wastewater by electrocoagulation. Sep. Sci. Technol. 2007, 42, 819–833. [Google Scholar] [CrossRef]
- Tezcan Un, U.; Topal, S.; Ates, F. Electrocoagulation of tissue paper wastewater and an evaluation of sludge for pyrolysis. Desalination Water Treat. 2016, 57, 28724–28733. [Google Scholar] [CrossRef]
- Eldars, F.M.S.E.; Ibrahim, M.A.; Gabr, A.M.E. Reduction of COD in water-based paint wastewater using three types of activated carbon. Desalination Water Treat. 2014, 52, 2975–2986. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-dimensional electrochemical process for wastewater treatment: a general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Jung, K.-W.; Hwang, M.-J.; Park, D.-S.; Ahn, K.-H. Performance evaluation and optimization of a fluidized three-dimensional electrode reactor combining pre-exposed granular activated carbon as a moving particle electrode for greywater treatment. Sep. Purif. Technol. 2015, 156, 414–423. [Google Scholar] [CrossRef]
- Xiong, Y.; Strunk, P.J.; Xia, H.; Zhu, X.; Karlsson, H.T. Treatment of dye wastewater containing acid orange II using a cell with three-phase three-dimensional electrode. Water Res. 2001, 35, 4226–4230. [Google Scholar] [CrossRef]











| Sample Name | pH | COD (mg/L) |
|---|---|---|
| Pb-Zn flotation wastewater | 11.90 | 434.7 |
| Pyrite concentrate wastewater | 9.11 | 245.3 |
| Pyrite tailing wastewater | 8.29 | 603.2 |
| Mixed wastewater | 11.83 | 424.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, G.; Ren, S.; Gao, Y.; Sun, W.; Gao, Z. Electrocoagulation: A Promising Method to Treat and Reuse Mineral Processing Wastewater with High COD. Water 2020, 12, 595. https://doi.org/10.3390/w12020595
Jing G, Ren S, Gao Y, Sun W, Gao Z. Electrocoagulation: A Promising Method to Treat and Reuse Mineral Processing Wastewater with High COD. Water. 2020; 12(2):595. https://doi.org/10.3390/w12020595
Chicago/Turabian StyleJing, Gaogui, Shuai Ren, Yuesheng Gao, Wei Sun, and Zhiyong Gao. 2020. "Electrocoagulation: A Promising Method to Treat and Reuse Mineral Processing Wastewater with High COD" Water 12, no. 2: 595. https://doi.org/10.3390/w12020595