Combining Tools from Edge-of-Field to In-Stream to Attenuate Reactive Nitrogen along Small Agricultural Waterways
Abstract
:1. Introduction
- managing small waterways to elicit effective change in the receiving environment,
- targeting local N export dynamics and underlying hydrological variability from agricultural land to waterways, and
- overcoming factors limiting N attenuation with suites of edge-of-field to waterway-based tools at multiple scales and locations. We also emphasize the need to
- encourage codevelopment of novel, effective, multiple-tool, multiple-scale waterway N attenuation approaches by scientists, practitioners, and farming communities to overcome the technical and practical challenges to managing N in agricultural landscapes.
2. Understanding and Managing for N Export Variability along Small Waterways
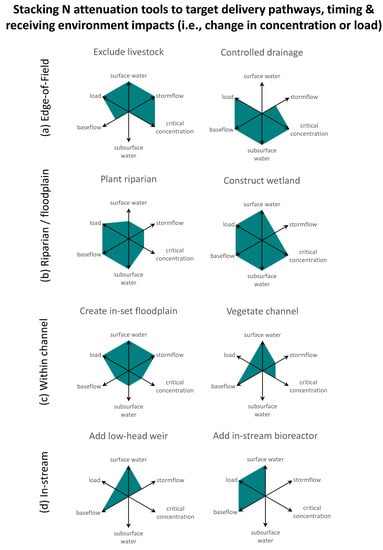
3. Expanding the N Toolbox to Boost Effectiveness from the Field Edge to In-Stream
4. Moving Forward: Codeveloping and Implementing N Attenuation Toolboxes on Working Farms
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harris, G.P.; Heathwaite, A.L. Why is achieving good ecological outcomes in rivers so difficult? Freshw. Biol. 2012, 57, 91–107. [Google Scholar] [CrossRef]
- Glibert, P.M. Eutrophication, harmful algae and biodiversity — challenging paradigms in a world of complex nutrient changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: a global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef]
- Graham, S.E.; O’Brien, J.M.; Burrell, T.K.; McIntosh, A.R. Aquatic macrophytes alter productivity-richness relationships in eutrophic stream food webs. Ecosphere 2015, 6, Article–89. [Google Scholar] [CrossRef]
- Dodds, W.K.; Oakes, R.M. Headwater influences on downstream water quality. Environ. Manag. 2008, 41, 367–377. [Google Scholar] [CrossRef] [Green Version]
- David, M.B.; Drinkwater, L.E.; McIsaac, G.F. Sources of nitrate yields in the Mississippi River Basin. J. Environ. Qual. 2010, 39, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Novotny, V. Diffuse pollution from agriculture — A worldwide outlook. Water Sci. Technol. 1999, 39, 1–13. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: a review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Monaghan, R.M.; de Klein, C.A.M.; Muirhead, R.W. Prioritisation of farm scale remediation efforts for reducing losses of nutrients and faecal indicator organisms to waterways: a case study of New Zealand dairy farming. J. Environ. Manage. 2008, 87, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Controlling eutrophication: nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.B.; Guse, B.; Fohrer, N. Assessing the impacts of Best Management Practices on nitrate pollution in an agricultural dominated lowland catchment considering environmental protection versus economic development. J. Environ. Manage. 2017, 196, 347–364. [Google Scholar] [CrossRef] [PubMed]
- McLellan, E.L.; Schilling, K.E.; Wolter, C.F.; Tomer, M.D.; Porter, S.A.; Magner, J.A.; Smith, D.R.; Prokopy, L.S. Right practice, right place: a conservation planning toolbox for meeting water quality goals in the Corn Belt. J. Soil Water Conserv. 2018, 73, 29A–34A. [Google Scholar] [CrossRef] [Green Version]
- Gascuel-Odoux, C.; Massa, F.; Durand, P.; Merot, P.; Troccaz, O.; Baudry, J.; Thenail, C. Framework and tools for agricultural landscape assessment relating to water quality protection. Environ. Manage. 2009, 43, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Tomer, M.D.; Porter, S.A.; James, D.E.; Boomer, K.M.B.; Kostel, J.A.; McLellan, E. Combining precision conservation technologies into a flexible framework to facilitate agricultural watershed planning. J. Soil Water Conserv. 2013, 68, 113A–120A. [Google Scholar] [CrossRef] [Green Version]
- McLellan, E.; Robertson, D.; Schilling, K.; Tomer, M.; Kostel, J.; Smith, D.; King, K. Reducing nitrogen export from the Corn Belt to the Gulf of Mexico: agricultural strategies for remediating hypoxia. JAWRA J. Am. Water Resour. Assoc. 2015, 51, 263–289. [Google Scholar] [CrossRef]
- Lien, E.; Magner, J. Engineered biosystem treatment trains: a review of agricultural nutrient sequestration. Invent. J. Res. Technol. Eng. Manag. 2017, 1, 1–8. [Google Scholar]
- Ranalli, A.J.; Macalady, D.L. The importance of the riparian zone and in-stream processes in nitrate attenuation in undisturbed and agricultural watersheds – a review of the scientific literature. J. Hydrol. 2010, 389, 406–415. [Google Scholar] [CrossRef]
- Dabney, S.M.; Moore, M.T.; Locke, M.A. Integrated management of in-field, edge-of-field, and after-field buffers. J. Am. Water Resour. Assoc. 2006, 42, 15–24. [Google Scholar] [CrossRef]
- Magner, J. Tailored Watershed Assessment and Integrated Management (TWAIM): a systems thinking approach. Water 2011, 3, 590–603. [Google Scholar] [CrossRef]
- McDowell, R.W.; Cox, N.; Snelder, T.H. Assessing the yield and load of contaminants with stream order: would policy requiring livestock to be fenced out of high-order streams decrease catchment contaminant loads? J. Environ. Qual. 2017, 46, 1038–1047. [Google Scholar] [CrossRef]
- Hughes, F.M.R.; Colston, A.; Mountford, J.O. Restoring riparian ecosystems: The challenge of accommodating variability and designing restoration trajectories. Ecol. Soc. 2005, 10, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Bernhardt, E.S.; Blaszczak, J.R.; Ficken, C.D.; Fork, M.L.; Kaiser, K.E.; Seybold, E.C. Control points in ecosystems: moving beyond the hot spot hot moment concept. Ecosystems 2017, 20, 665–682. [Google Scholar] [CrossRef]
- Abbott, B.W.; Gruau, G.; Zarnetske, J.P.; Moatar, F.; Barbe, L.; Thomas, Z.; Fovet, O.; Kolbe, T.; Gu, S.; Pierson-Wickmann, A.-C.; et al. Unexpected spatial stability of water chemistry in headwater stream networks. Ecol. Lett. 2017, 21, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.S.; Palmer, M.A.; Richardson, D.C.; Filoso, S.; Bernhardt, E.S.; Bledsoe, B.P.; Doyle, M.W.; Groffman, P.M.; Hassett, B.A.; Kaushal, S.S.; et al. Stream restoration strategies for reducing river nitrogen loads. Front. Ecol. Environ. 2008, 6, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Peterson, B.J.; Wollheim, W.M.; Mulholland, P.J.; Webster, J.R.; Meyer, J.L.; Tank, J.L.; Martí, E.; Bowden, W.B.; Valett, H.M.; Hershey, A.E.; et al. Control of nitrogen export from watersheds by headwater streams. Science 2001, 292, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Royer, T.V.; Tank, J.L.; David, M.B. Transport and fate of nitrate in headwater agricultural streams in Illinois. J. Environ. Qual. 2004, 33, 1296–1304. [Google Scholar] [CrossRef]
- Bernot, M.J.; Tank, J.L.; Royer, T.V.; David, M.B. Nutrient uptake in streams draining agricultural catchments of the midwestern United States. Freshw. Biol. 2006, 51, 499–509. [Google Scholar] [CrossRef]
- Kröger, R.; Holland, M.M.; Moore, M.T.; Cooper, C.M. Hydrological variability and agricultural drainage ditch inorganic nitrogen reduction capacity. J. Environ. Qual. 2007, 36, 1646–1652. [Google Scholar] [CrossRef] [Green Version]
- Ensign, S.H.; Doyle, M.W. In-channel transient storage and associated nutrient retention: Evidence from experimental manipulations. Limnol. Oceanogr. 2005, 50, 1740–1751. [Google Scholar] [CrossRef] [Green Version]
- Kröger, R.; Moore, M.T.; Farris, J.L.; Gopalan, M. Evidence for the use of low-grade weirs in drainage ditches to improve nutrient reductions from agriculture. Water. Air. Soil Pollut. 2011, 221, 223–234. [Google Scholar] [CrossRef]
- Alexander, R.B.; Boyer, E.W.; Smith, R.A.; Schwarz, G.E.; Moore, R.B. The role of headwater streams in downstream water quality. JAWRA J. Am. Water Resour. Assoc. 2007, 43, 41–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, G. Improving restoration practice by deriving appropriate techniques from analysing the spatial organization of river networks. Limnol.-Ecol. Manag. Inland Waters 2014, 45, 50–60. [Google Scholar] [CrossRef]
- Royer, T.V.; David, M.B.; Gentry, L.E. Timing of riverine export of nitrate and phosphorus from agricultural watersheds in Illinois: implications for reducing nutrient loading to the Mississippi River. Environ. Sci. Technol. 2006, 40, 4126–4131. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; David, M.B.; Lemke, D.W.; Jaynes, D.B. Understanding nutrient fate and transport, including the importance of hydrology in determining field losses. In Final Report: Gulf Hypoxia and Local Water Quality Concerns Workshop; Upper Mississippi River Subbasin Hypoxia Nutrient Committee, Ed.; American Society of Agricultural and Biological Engineers: St. Joseph, Michigan, 2008; pp. 1–17. [Google Scholar]
- Monaghan, R.M.; Smith, L.C.; Muirhead, R.W. Pathways of contaminant transfers to water from an artificially-drained soil under intensive grazing by dairy cows. Agric. Ecosyst. Environ. 2016, 220, 76–88. [Google Scholar] [CrossRef]
- Liu, J.; Baulch, H.M.; Macrae, M.L.; Wilson, H.F.; Elliott, J.A.; Bergström, L.; Glenn, A.J.; Vadas, P.A. Agricultural water quality in cold climates: processes, drivers, management options, and research needs. J. Environ. Qual. 2019, 48, 792. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.R.; Buda, A.R.; Elliott, H.A.; Singha, K.; Hamlett, J. Influence of riparian seepage zones on nitrate variability in two agricultural headwater streams. JAWRA J. Am. Water Resour. Assoc. 2015, 51, 883–897. [Google Scholar] [CrossRef]
- Williams, M.R.; King, K.W.; Fausey, N.R. Contribution of tile drains to basin discharge and nitrogen export in a headwater agricultural watershed. Agric. Water Manag. 2015, 158, 42–50. [Google Scholar] [CrossRef]
- Goeller, B.C.; Febria, C.M.; Warburton, H.J.; Hogsden, K.L.; Collins, K.E.; Devlin, H.S.; Harding, J.S.; McIntosh, A.R. Springs drive downstream nitrate export from artificially-drained agricultural headwater catchments. Sci. Total Environ. 2019, 671, 119–128. [Google Scholar] [CrossRef]
- Jaynes, D.B.; Isenhart, T.M. Reconnecting tile drainage to riparian buffer hydrology for enhanced nitrate removal. J. Environ. Qual. 2014, 43, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Jordan, S.J.; Stoffer, J.; Nestlerode, J.A. Wetlands as sinks for reactive nitrogen at continental and global scales: a meta-analysis. Ecosystems 2011, 14, 144–155. [Google Scholar] [CrossRef]
- Bauwe, A.; Tiemeyer, B.; Kahle, P.; Lennartz, B. Classifying hydrological events to quantify their impact on nitrate leaching across three spatial scales. J. Hydrol. 2015, 531, 589–601. [Google Scholar] [CrossRef]
- Robinson, D.A.; Binley, A.; Crook, N.; Day-Lewis, F.D.; Ferré, T.P.A.; Grauch, V.J.S.; Knight, R.; Knoll, M.; Lakshmi, V.; Miller, R.; et al. Advancing process-based watershed hydrological research using near-surface geophysics: a vision for, and review of, electrical and magnetic geophysical methods. Hydrol. Process. 2008, 22, 3604–3635. [Google Scholar] [CrossRef]
- Blume, T.; van Meerveld, H.J. From hillslope to stream: methods to investigate subsurface connectivity. Wiley Interdiscip. Rev. Water 2015, 2, 177–198. [Google Scholar] [CrossRef] [Green Version]
- Rittenburg, R.A.; Squires, A.L.; Boll, J.; Brooks, E.S.; Easton, Z.M.; Steenhuis, T.S. Agricultural BMP effectiveness and dominant hydrological flow paths: concepts and a review. JAWRA J. Am. Water Resour. Assoc. 2015, 51, 305–329. [Google Scholar] [CrossRef]
- McDonnell, J.J. Are all runoff processes the same? Hydrol. Process. 2013, 27, 4103–4111. [Google Scholar] [CrossRef]
- Covino, T. Hydrologic connectivity as a framework for understanding biogeochemical flux through watersheds and along fluvial networks. Geomorphology 2017, 277, 133–144. [Google Scholar] [CrossRef]
- Deakin, J.; Flynn, R.; Archbold, M.; Daly, D.; O’Brien, R.; Orr, A.; Misstear, N. Understanding pathways transferring nutrients to streams: review of a major Irish study and its implications for determining water quality management strategies. Biol. Environ. Proc. R. Ir. Acad. 2016, 116B, 233–243. [Google Scholar] [CrossRef]
- Giri, S.; Nejadhashemi, A.P.; Woznicki, S.; Zhang, Z. Analysis of best management practice effectiveness and spatiotemporal variability based on different targeting strategies. Hydrol. Process. 2014, 28, 431–445. [Google Scholar] [CrossRef]
- Pearce, N.J.T.; Yates, A.G. Intra-annual variation of the association between agricultural best management practices and stream nutrient concentrations. Sci. Total Environ. 2017, 586, 1124–1134. [Google Scholar] [CrossRef]
- Williams, M.R.; King, K.W.; Penn, C.J. Integrating temporal inequality into conservation planning to improve practice design and efficacy. JAWRA J. Am. Water Resour. Assoc. 2018, 54, 1039–1054. [Google Scholar] [CrossRef]
- Hill, A.R. Groundwater nitrate removal in riparian buffer zones: a review of research progress in the past 20 years. Biogeochemistry 2019, 143, 347–369. [Google Scholar] [CrossRef]
- Goeller, B.C.; Burbery, L.F.; Febria, C.M.; Collins, K.E.; Burrows, N.J.; Simon, K.S.; Harding, J.S.; McIntosh, A.R. Capacity for bioreactors and riparian rehabilitation to enhance nitrate attenuation in agricultural streams. Ecol. Eng. 2019, 134, 65–77. [Google Scholar] [CrossRef]
- Balestrini, R.; Sacchi, E.; Tidili, D.; Delconte, C.A.; Buffagni, A. Factors affecting agricultural nitrogen removal in riparian strips: Examples from groundwater-dependent ecosystems of the Po Valley (Northern Italy). Agric. Ecosyst. Environ. 2016, 221, 132–144. [Google Scholar] [CrossRef]
- Tanner, C.C.; Kadlec, R.H. Influence of hydrological regime on wetland attenuation of diffuse agricultural nitrate losses. Ecol. Eng. 2013, 56, 79–88. [Google Scholar] [CrossRef]
- Goeller, B.C.; Febria, C.M.; Harding, J.S.; McIntosh, A.R. Thinking beyond the bioreactor box: incorporating stream ecology into edge-of-field nitrate management. J. Environ. Qual. 2016, 45, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Viaud, V.; Merot, P.; Baudry, J. Hydrochemical buffer assessment in agricultural landscapes: from local to catchment scale. Environ. Manage. 2004, 34, 559–573. [Google Scholar] [CrossRef]
- Stamm, C.; Jarvie, H.P.; Scott, T. What’s more important for managing phosphorus: loads, concentrations or both? Environ. Sci. Technol. 2014, 48, 23–24. [Google Scholar] [CrossRef]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of U.S. freshwaters: analysis of potential economic damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Davies-Colley, R.J.; Nagels, J.W.; Smith, R.A.; Young, R.G.; Phillips, C.J. Water quality impact of a dairy cow herd crossing a stream. N. Z. J. Mar. Freshw. Res. 2004, 38, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.O.; Tanner, C.C.; McKergow, L.A.; Sukias, J.P.S. Unrestricted dairy cattle grazing of a pastoral headwater wetland and its effect on water quality. Agric. Water Manag. 2016, 165, 72–81. [Google Scholar] [CrossRef]
- O’Callaghan, P.; Kelly-Quinn, M.; Jennings, E.; Antunes, P.; O’Sullivan, M.; Fenton, O.; hUallacháin, D.Ó. The environmental impact of cattle access to watercourses: a review. J. Environ. Qual. 2019, 48, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Evans, R.O.; Smith, J.T. Effect of controlled drainage and vegetative buffers on drainage water quality from wastewater irrigated fields. J. Irrig. Drain. Eng. 2006, 132, 159–170. [Google Scholar] [CrossRef]
- Woli, K.P.; David, M.B.; Cooke, R.A.; McIsaac, G.F.; Mitchell, C.A. Nitrogen balance in and export from agricultural fields associated with controlled drainage systems and denitrifying bioreactors. Ecol. Eng. 2010, 36, 1558–1566. [Google Scholar] [CrossRef]
- Bonaiti, G.; Borin, M. Efficiency of controlled drainage and subirrigation in reducing nitrogen losses from agricultural fields. Agric. Water Manag. 2010, 98, 343–352. [Google Scholar] [CrossRef]
- Carstensen, M.V.; Børgesen, C.D.; Ovesen, N.B.; Poulsen, J.R.; Hvid, S.K.; Kronvang, B. Controlled drainage as a targeted mitigation measure for nitrogen and phosphorus. J. Environ. Qual. 2019, 48, 677–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edwards, C.L.; Shannon, R.D.; Jarrett, A.R. Sedimentation basin retention efficiencies for sediment, nitrogen, and phosphorus from simulated agricultural runoff. Trans. ASAE 1999, 403–409. [Google Scholar] [CrossRef]
- Wang, X.H.; Yin, C.Q.; Shan, B.Q. The role of diversified landscape buffer structures for water quality improvement in an agricultural watershed, North China. Agric. Ecosyst. Environ. 2005, 107, 381–396. [Google Scholar] [CrossRef]
- Fiener, P.; Auerswald, K.; Weigand, S. Managing erosion and water quality in agricultural watersheds by small detention ponds. Agric. Ecosyst. Environ. 2005, 110, 132–142. [Google Scholar] [CrossRef]
- Chrétien, F.; Gagnon, P.; Thériault, G.; Guillou, M. Performance analysis of a wet-retention pond in a small agricultural catchment. J. Environ. Eng. 2016, 142, 04016005. [Google Scholar] [CrossRef]
- Smith, C.M. Riparian pasture retirement effects on sediment, phosphorus, and nitrogen in channellised surface run-off from pastures. N. Z. J. Mar. Freshw. Res. 1989, 23, 139–146. [Google Scholar] [CrossRef]
- Daniels, R.B.; Gilliam, J.W. Sediment and chemical load reduction by grass and riparian filters. Soil Sci. Soc. Am. J. 1996, 60, 246–251. [Google Scholar] [CrossRef]
- Clausen, J.C.; Guillard, K.; Sigmund, C.M.; Dors, K.M. Water quality changes from riparian buffer restoration in Connecticut. J. Environ. Qual. 2000, 29, 1751–1761. [Google Scholar] [CrossRef] [Green Version]
- Long, L.M.; Schipper, L.A.; Bruesewitz, D.A. Long-term nitrate removal in a denitrification wall. Agric. Ecosyst. Environ. 2011, 140, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.A.; Clark, M.W. Evaluation of a denitrification wall to reduce surface water nitrogen loads. J. Environ. Qual. 2012, 41, 724–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorman, T.B.; Tomer, M.D.; Smith, D.R.; Jaynes, D.B. Evaluating the potential role of denitrifying bioreactors in reducing watershed-scale nitrate loads: a case study comparing three Midwestern (USA) watersheds. Ecol. Eng. 2015, 75, 441–448. [Google Scholar] [CrossRef]
- Addy, K.; Gold, A.J.; Christianson, L.E.; David, M.B.; Schipper, L.A.; Ratigan, N.A. Denitrifying bioreactors for nitrate removal: a meta-analysis. J. Environ. Qual. 2016, 45, 873–881. [Google Scholar] [CrossRef]
- Kovacic, D.A.; David, M.B.; Gentry, L.E.; Starks, K.M.; Cooke, R.A. Effectiveness of constructed wetlands in reducing nitrogen and phosphorus export from agricultural tile drainage. J. Environ. Qual. 2000, 29, 1262–1274. [Google Scholar] [CrossRef] [Green Version]
- Tanner, C.C.; Sukias, J.P.S.; Yates, C.R. New Zealand Guidelines: Constructed Wetland Treatment of tile Drainage; National Institute of Water & Atmospheric Research Ltd.: Hamilton, New Zealand, 2010. [Google Scholar]
- Tanner, C.C.; Sukias, J.P.S. Multiyear nutrient removal performance of three constructed wetlands intercepting tile drain flows from grazed pastures. J. Environ. Qual. 2011, 40, 620–633. [Google Scholar] [CrossRef]
- Hefting, M.M.; van den Heuvel, R.N.; Verhoeven, J.T.A. Wetlands in agricultural landscapes for nitrogen attenuation and biodiversity enhancement: opportunities and limitations. Ecol. Eng. 2013, 56, 5–13. [Google Scholar] [CrossRef]
- Jaynes, D.B.; Isenhart, T.M. Performance of saturated riparian buffers in Iowa, USA. J. Environ. Qual. 2019, 48, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Tomer, M.D.; Jaynes, D.B.; Porter, S.A.; James, D.E.; Isenhart, T.M. Identifying riparian zones best suited to installation of saturated buffers: a preliminary multi-watershed assessment. In Precision Conservation: Geospatial Techniques for Agricultural and Natural Resources Conservation; Delgado, J., Sassenrath, G., Mueller, T., Eds.; Agronomy Monographs; ASA, CSSA, and SSSA: Madison, Wisconsin, USA, 2017. [Google Scholar]
- Davis, M.P.; Groh, T.A.; Jaynes, D.B.; Parkin, T.B.; Isenhart, T.M. Nitrous oxide emissions from saturated riparian buffers: are we trading a water quality problem for an air quality problem? J. Environ. Qual. 2018, 48, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fennessy, M.S.; Cronk, J.K. The effectiveness and restoration potential of riparian ecotones for the management of nonpoint source pollution, particularly nitrate. Crit. Rev. Environ. Sci. Technol. 1997, 27, 285–317. [Google Scholar] [CrossRef]
- Stutter, M.; Kronvang, B.; Ó hUallacháin, D.; Rozemeijer, J. Current insights into the effectiveness of riparian management, attainment of multiple benefits, and potential technical enhancements. J. Environ. Qual. 2019, 48, 236–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valkama, E.; Usva, K.; Saarinen, M.; Uusi-Kämppä, J. A meta-analysis on nitrogen retention by buffer zones. J. Environ. Qual. 2019, 48, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Welsh, M.K.; McMillan, S.K.; Vidon, P.G. Denitrification along the stream-riparian continuum in restored and unrestored agricultural streams. J. Environ. Qual. 2017, 46, 1010–1019. [Google Scholar] [CrossRef]
- Webster, A.J.; Groffman, P.M.; Cadenasso, M.L. Controls on denitrification potential in nitrate-rich waterways and riparian zones of an irrigated agricultural setting. Ecol. Appl. 2018, 28, 1055–1067. [Google Scholar] [CrossRef]
- Kasahara, T.; Hill, A.R. Lateral hyporheic zone chemistry in an artificially constructed gravel bar and a re-meandered stream channel, Southern Ontario, Canada. JAWRA J. Am. Water Resour. Assoc. 2007, 43, 1257–1269. [Google Scholar] [CrossRef]
- Weigelhofer, G.; Welti, N.; Hein, T. Limitations of stream restoration for nitrogen retention in agricultural headwater streams. Ecol. Eng. 2013, 60, 224–234. [Google Scholar]
- Roley, S.S.; Tank, J.L.; Stephen, M.L.; Johnson, L.T.; Beaulieu, J.J.; Witter, J.D. Floodplain restoration enhances denitrification and reach-scale nitrogen removal in an agricultural stream. Ecol. Appl. 2012, 22, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Mahl, U.H.; Tank, J.L.; Roley, S.S.; Davis, R.T. Two-stage ditch floodplains enhance N-removal capacity and reduce turbidity and dissolved P in agricultural streams. JAWRA J. Am. Water Resour. Assoc. 2015, 51, 923–940. [Google Scholar] [CrossRef]
- Hodaj, A.; Bowling, L.C.; Frankenberger, J.R.; Chaubey, I. Impact of a two-stage ditch on channel water quality. Agric. Water Manag. 2017, 192, 126–137. [Google Scholar] [CrossRef]
- Hanrahan, B.R.; Tank, J.L.; Dee, M.M.; Trentman, M.T.; Berg, E.M.; McMillan, S.K. Restored floodplains enhance denitrification compared to naturalized floodplains in agricultural streams. Biogeochemistry 2018, 1–19. [Google Scholar] [CrossRef]
- Riley, W.D.; Potter, E.C.E.; Biggs, J.; Collins, A.L.; Jarvie, H.P.; Jones, J.I.; Kelly-Quinn, M.; Ormerod, S.J.; Sear, D.A.; Wilby, R.L.; et al. Small water bodies in Great Britain and Ireland: ecosystem function, human-generated degradation, and options for restorative action. Sci. Total Environ. 2018, 645, 1598–1616. [Google Scholar] [CrossRef] [PubMed]
- Booman, G.C.; Laterra, P. Channelizing streams for agricultural drainage impairs their nutrient removal capacity. J. Environ. Qual. 2019, 48, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Soana, E.; Bartoli, M.; Milardi, M.; Fano, E.A.; Castaldelli, G. An ounce of prevention is worth a pound of cure: managing macrophytes for nitrate mitigation in irrigated agricultural watersheds. Sci. Total Environ. 2019, 647, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Castaldelli, G.; Soana, E.; Racchetti, E.; Vincenzi, F.; Fano, E.A.; Bartoli, M. Vegetated canals mitigate nitrogen surplus in agricultural watersheds. Agric. Ecosyst. Environ. 2015, 212, 253–262. [Google Scholar] [CrossRef]
- Soana, E.; Balestrini, R.; Vincenzi, F.; Bartoli, M.; Castaldelli, G. Mitigation of nitrogen pollution in vegetated ditches fed by nitrate-rich spring waters. Agric. Ecosyst. Environ. 2017, 243, 74–82. [Google Scholar] [CrossRef]
- Kasahara, T.; Hill, A.R. Effects of riffle step restoration on hyporheic zone chemistry in N-rich lowland streams. Can. J. Fish. Aquat. Sci. 2006, 63, 120–133. [Google Scholar] [CrossRef]
- Lautz, L.K.; Fanelli, R.M. Seasonal biogeochemical hotspots in the streambed around restoration structures. Biogeochemistry 2008, 91, 85–104. [Google Scholar] [CrossRef]
- Kröger, R.; Scott, J.T.; Czarnecki, J.M.P. Denitrification potential of low-grade weirs and agricultural drainage ditch sediments in the Lower Mississippi Alluvial Valley. Ecol. Eng. 2014, 73, 168–175. [Google Scholar] [CrossRef]
- Faust, D.R.; Kröger, R.; Moore, M.T.; Rush, S.A. Management practices used in agricultural drainage ditches to reduce Gulf of Mexico hypoxia. Bull. Environ. Contam. Toxicol. 2017, 100, 32–40. [Google Scholar] [CrossRef]
- Lazar, J.G.; Gold, A.J.; Addy, K.; Mayer, P.M.; Forshay, K.J.; Groffman, P.M. Instream large wood: denitrification hotspots with low N2O production. JAWRA J. Am. Water Resour. Assoc. 2014, 50, 615–625. [Google Scholar] [CrossRef]
- Shipitalo, M.J.; Bonta, J.V.; Dayton, E.A.; Owens, L.B. Impact of grassed waterways and compost filter socks on the quality of surface runoff from corn fields. J. Environ. Qual. 2010, 39, 1009–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faust, D.R.; Kröger, R.; Miranda, L.E.; Rush, S.A. Nitrate removal from agricultural drainage ditch sediments with amendments of organic carbon: potential for an innovative best management practice. Water. Air. Soil Pollut. 2016, 10, 378–387. [Google Scholar] [CrossRef]
- O’Brien, J.M.; Warburton, H.J.; Graham, S.E.; Franklin, H.M.; Febria, C.M.; Hogsden, K.L.; Harding, J.S.; McIntosh, A.R. Leaf litter additions enhance stream metabolism, denitrification, and restoration prospects for agricultural catchments. Ecosphere 2017, 8, e02018. [Google Scholar] [CrossRef]
- Nifong, R.L.; Taylor, J.M.; Moore, M.T. Mulch-derived organic carbon stimulates high denitrification fluxes from agricultural ditch sediments. J. Environ. Qual. 2019, 48, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Robertson, W.D.; Merkley, L.C. In-stream bioreactor for agricultural nitrate treatment. J. Environ. Qual. 2009, 38, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Elgood, Z.; Robertson, W.D.; Schiff, S.L.; Elgood, R. Nitrate removal and greenhouse gas production in a stream-bed denitrifying bioreactor. Ecol. Eng. 2010, 36, 1575–1580. [Google Scholar] [CrossRef]
- Pfannerstill, M.; Kühling, I.; Hugenschmidt, C.; Trepel, M.; Fohrer, N. Reactive ditches: A simple approach to implement denitrifying wood chip bioreactors to reduce nitrate exports into aquatic ecosystems? Environ. Earth Sci. 2016, 75, 1063–1073. [Google Scholar] [CrossRef]
- Sarris, T.S.; Burbery, L.F. Stochastic multi-objective performance optimization of an in-stream woodchip denitrifying bioreactor. Ecol. Eng. 2018, 124, 38–50. [Google Scholar] [CrossRef]
- Newcomer Johnson, T.; Kaushal, S.; Mayer, P.; Smith, R.; Sivirichi, G. Nutrient retention in restored streams and rivers: a global review and synthesis. Water 2016, 8, 116. [Google Scholar] [CrossRef] [Green Version]
- Burgin, A.J.; Hamilton, S.K. Have we overemphasized the role of denitrification in aquatic ecosystems? a review of nitrate removal pathways. Front. Ecol. Environ. 2007, 5, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Schoumans, O.F.; Chardon, W.J.; Bechmann, M.E.; Gascuel-Odoux, C.; Hofman, G.; Kronvang, B.; Rubæk, G.H.; Ulén, B.; Dorioz, J.-M. Mitigation options to reduce phosphorus losses from the agricultural sector and improve surface water quality: A review. Sci. Total Environ. 2014, 468–469, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Roley, S.S.; Tank, J.L.; Tyndall, J.C.; Witter, J.D. How cost-effective are cover crops, wetlands, and two-stage ditches for nitrogen removal in the Mississippi River Basin? Water Resour. Econ. 2016, 15, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Seitzinger, S.; Harrison, J.A.; Böhlke, J.K.; Bouwman, A.F.; Lowrance, R.; Peterson, B.; Tobias, C.; Drecht, G.V. Denitrification across landscapes and waterscapes: a synthesis. Ecol. Appl. 2006, 16, 2064–2090. [Google Scholar] [CrossRef] [Green Version]
- Filoso, S.; Palmer, M.A. Assessing stream restoration effectiveness at reducing nitrogen export to downstream waters. Ecol. Appl. 2011, 21, 1989–2006. [Google Scholar] [CrossRef]
- Doyle, M.W.; Shields, F.D. Compensatory mitigation for streams under the Clean Water Act: reassessing science and redirecting policy. JAWRA J. Am. Water Resour. Assoc. 2012, 48, 494–509. [Google Scholar] [CrossRef]
- Lammers, R.W.; Bledsoe, B.P. What role does stream restoration play in nutrient management? Crit. Rev. Environ. Sci. Technol. 2017, 47, 335–371. [Google Scholar] [CrossRef]
- Collier, K.J.; Cooper, A.B.; Davies-Colley, R.J.; Rutherford, J.C.; Smith, C.M.; Williamson, R.B. Managing Riparian Zones: A Contribution to Protecting New Zealand’s Rivers and Streams; Department of Conservation: Wellington, New Zealand, 1995.
- Ellawala Kankanamge, C.; Matheson, F.E.; Riis, T. Shading constrains the growth of invasive submerged macrophytes in streams. Aquat. Bot. 2019, 158, 1–7. [Google Scholar] [CrossRef]
- Burrell, T.K.; O’Brien, J.M.; Graham, S.E.; Simon, K.S.; Harding, J.S.; McIntosh, A.R. Riparian shading mitigates stream eutrophication in agricultural catchments. Freshw. Sci. 2014, 33, 73–84. [Google Scholar] [CrossRef]
- Halliday, S.J.; Skeffington, R.A.; Wade, A.J.; Bowes, M.J.; Read, D.S.; Jarvie, H.P.; Loewenthal, M. Riparian shading controls instream spring phytoplankton and benthic algal growth. Env. Sci Process. Impacts 2016, 18, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meals, D.W.; Dressing, S.A.; Davenport, T.E. Lag time in water quality response to best management practices: a review. J. Environ. Qual. 2010, 39, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.E.; Baker, M.E. Cropland riparian buffers throughout Chesapeake Bay watershed: spatial patterns and effects on nitrate loads delivered to streams. JAWRA J. Am. Water Resour. Assoc. 2014, 50, 696–712. [Google Scholar] [CrossRef]
- Mander, Ü.; Tournebize, J.; Tonderski, K.; Verhoeven, J.T.A.; Mitsch, W.J. Planning and establishment principles for constructed wetlands and riparian buffer zones in agricultural catchments. Ecol. Eng. 2017, 103, 296–300. [Google Scholar] [CrossRef]
- Pearce, N.; Yates, A. Agricultural best management practice abundance and location does not influence stream ecosystem function or water quality in the summer season. Water 2015, 7, 6861–6876. [Google Scholar] [CrossRef] [Green Version]
- Lawson, D.M.; Hall, K.R.; Yung, L.; Enquist, C.A. Building translational ecology communities of practice: insights from the field. Front. Ecol. Environ. 2017, 15, 569–577. [Google Scholar] [CrossRef] [Green Version]
- David, M.B.; Flint, C.G.; Gentry, L.E.; Dolan, M.K.; Czapar, G.F.; Cooke, R.A.; Lavaire, T. Navigating the socio-bio-geo-chemistry and engineering of nitrogen management in two Illinois tile-drained watersheds. J. Environ. Qual. 2015, 44, 368–381. [Google Scholar] [CrossRef] [Green Version]
- Beechie, T.; Pess, G.; Roni, P.; Giannico, G. Setting river restoration priorities: a review of approaches and a general protocol for identifying and prioritizing actions. N. Am. J. Fish. Manag. 2008, 28, 891–905. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Palmer, M.A. River restoration: the fuzzy logic of repairing reaches to reverse catchment scale degradation. Ecol. Appl. 2011, 21, 1926–1931. [Google Scholar] [CrossRef]
- Hermoso, V.; Pantus, F.; Olley, J.; Linke, S.; Mugodo, J.; Lea, P. Systematic planning for river rehabilitation: integrating multiple ecological and economic objectives in complex decisions: freshwater systematic rehabilitation planning. Freshw. Biol. 2012, 57, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, H.M.; Closs, G.P.; Townsend, C.R. Stream ecosystem health outcomes of providing information to farmers and adoption of best management practices. J. Appl. Ecol. 2007, 44, 1106–1115. [Google Scholar] [CrossRef]
- Hallett, L.M.; Morelli, T.L.; Gerber, L.R.; Moritz, M.A.; Schwartz, M.W.; Stephenson, N.L.; Tank, J.L.; Williamson, M.A.; Woodhouse, C.A. Navigating translational ecology: creating opportunities for scientist participation. Front. Ecol. Environ. 2017, 15, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Enquist, C.A.; Jackson, S.T.; Garfin, G.M.; Davis, F.W.; Gerber, L.R.; Littell, J.A.; Tank, J.L.; Terando, A.J.; Wall, T.U.; Halpern, B.; et al. Foundations of translational ecology. Front. Ecol. Environ. 2017, 15, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Winfield, I.J. Meeting across the river: from science to impact. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 607–610. [Google Scholar] [CrossRef]
- Schilling, K.E.; Streeter, M.T.; St. Clair, M.; Meissen, J. Subsurface nutrient processing capacity in agricultural roadside ditches. Sci. Total Environ. 2018, 637, 470–479. [Google Scholar] [CrossRef]

| Location | N Attenuation Tool | Baseflow Versus Stormflow Attenuation | Intercepted Hydraulic Flow Pathway | Effect in the Receiving Environment | Benefits and Disbenefits | Example |
|---|---|---|---|---|---|---|
| Edge-of-field | Exclude livestock | baseflow, stormflow | surface drains/streams, standing water, surface runoff | decreased load | B: reduced stock losses, aesthetics D: fence maintenance, alternative drinking water sources, and potential weed management issues | [60,61,62] |
| Redirect subsurface drainage (e.g., controlled drainage) | baseflow, stormflow | tile drains | decreased load, decreased concentration peaks | B: soil water storage, flood attenuation D: requires active management | [63,64,65,66] | |
| Detain water (e.g., retention/detention bunds, ponds, or basins) | stormflow | standing surface water, surface runoff | decreased load, decreased concentration peaks | B: soil water storage, flood attenuation, can reduce drain clearance costs D: requires active management | [67,68,69,70] | |
| Retain grass filter strips and swales | stormflow | surface runoff, surface drains | decreased load, decreased concentration peaks | D: potential weed management issues | [71,72,73] | |
| Install denitrification beds or walls | baseflow | tile drains, subsurface flow | decreased load | B: little reduction of productive land D: initial flush of organic carbon, anoxic effluent, dissolved phosphorus release under anoxia, greenhouse gas production | [74,75,76,77] | |
| Riparian buffer/floodplain | Construct or enhance wetlands | baseflow, stormflow | floods, surface drains, tile drains, standing surface water, subsurface flow | decreased load, decreased concentration peaks | B: able to cope with fluctuating water levels, stock water supply, waterfowl habitat, flood attenuation, recreation, biodiversity value, landscape aesthetics D: source of avian E.coli, dissolved phosphorus release under anoxia, greenhouse gas production, nutrient impacts on natural wetland ecology | [78,79,80,81] |
| Disconnect tile drains to saturate riparian buffer | baseflow | tile drains | decreased load | B: soil water storage, flood attenuation D: requires active management | [40,82,83,84] | |
| Plant riparian vegetation | baseflow | surface flow, subsurface flow | decreased load, decreased concentration peaks | B: channel shading, improved aquatic habitat, wood and leaf supply to stream, recreation, harvesting of biomass, biodiversity value, landscape aesthetics D: requires some active vegetation management, shading might suppress in-stream nutrient uptake | [85,86,87] | |
| Within channel margins | Reshape stream banks | baseflow, stormflow | subsurface flow, surface drains/streams, floods | decreased load, decreased concentration peaks | B: able to cope with fluctuating water levels | [24,88,89] |
| Create meander bends | baseflow | surface drains/streams | decreased load, decreased concentration peaks | B: able to cope with fluctuating water levels, flood attenuation, biodiversity value, landscape aesthetics | [24,90,91] | |
| Create inset floodplains (e.g., two-stage channels) | baseflow, stormflow | surface drains/streams, tile drains, floods | decreased load, decreased concentration peaks | B: able to cope with fluctuating water levels, flood attenuation, biodiversity value | [92,93,94,95] | |
| Widen channel | baseflow, stormflow | surface drains/streams, floods | decreased load | B: able to cope with fluctuating water levels, flood attenuation D: potential sedimentation issues, weed management | [24,96,97] | |
| Vegetate channel or maintain in-ditch vegetation | baseflow | surface drains/streams | decreased load, decreased concentration peaks | B: forage crop for stock, biodiversity value D: potential heightened flood risk, sedimentation issues, requires active management | [98,99,100] | |
| In-stream | Add in-stream geomorphic features (e.g., boulders, riffles) | baseflow | surface drains/streams | decreased load | B: biodiversity value, landscape aesthetics D: heightened winter flood risk | [24,101,102] |
| Add debris dams/ low-grade weirs | baseflow, stormflow | surface drains/streams, floods | decreased load, decreased concentration peaks | B: able to cope with fluctuating water levels D: heightened winter flood risk | [30,103,104] | |
| Add large woody debris | baseflow | surface drains/streams | decreased load | B: biodiversity value, landscape aesthetics D: heightened winter flood risk | [24,29,105] | |
| Add organic matter (e.g., leaves, small wood) | baseflow | surface drains/streams | decreased load | B: biodiversity value D: heightened winter flood risk | [106,107,108,109] | |
| Add in-stream bioreactors | baseflow | surface drains/streams | decreased load | D: initial flush of organic carbon, anoxic effluent, dissolved phosphorus release under anoxia, greenhouse gas production | [110,111,112,113] |
| Location | N Attenuation Tool | Dominant Attenuation Mechanisms 1 | Increases Filtering, Deposition or Adsorption | Increases Water Retention Time | Enhances Surface-to-Groundwater Exchange | Increases Surface Area-to-Volume Ratio (Contact with Soil and Benthos) | Promotes Contact with Vegetation or Algae and Organic Soils or Substrates |
|---|---|---|---|---|---|---|---|
| Edge-of-field | Exclude livestock | P | + + + | ||||
| Redirect subsurface drainage (e.g., controlled drainage) | M, B | + + + | + + | + + | |||
| Detain water (e.g., retention/detention bunds, ponds, or basins) | P, M | + + + | + + + | + + | + + | + | |
| Retain grass filter strips and swales | P, M, B | + + + | + | + | + + | + + | |
| Install denitrification beds or walls | M, P | + | + + | + + + | |||
| Riparian buffer/floodplain | Construct or enhance wetlands | M, B, P | + + | + + + | + | + + | + + + |
| Disconnect tile drains to saturate riparian buffer | M, B | + + | + + + | + | + + | + + + | |
| Plant riparian vegetation | M, B, P | + + | + | + + + | |||
| Within channel margins | Reshape stream banks | B, M, P | + | + | + | + | |
| Create meander bends | M, B | + | + | + | + + | ||
| Create inset floodplains (e.g., two-stage channels) | M, B, P | + | + | + | + + | + + + | |
| Widen channel | M, B | + + | + | + + | |||
| Vegetate channel or maintain in-ditch vegetation | M, B | + | + | + + + | |||
| In-stream | Add in-stream geomorphic features (e.g., boulders, riffles) | M, B, P | + + | + | + | + | |
| Add debris dams/low-grade weirs | M, B, P | + + | + + | + | |||
| Add large woody debris | M, B | + + | + + | + | + | ||
| Add organic matter (e.g., leaves, small wood) | M, B | + + | + | + | |||
| Add in-stream bioreactors | M, P | + + + | + + + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goeller, B.C.; Febria, C.M.; McKergow, L.A.; Harding, J.S.; Matheson, F.E.; Tanner, C.C.; McIntosh, A.R. Combining Tools from Edge-of-Field to In-Stream to Attenuate Reactive Nitrogen along Small Agricultural Waterways. Water 2020, 12, 383. https://doi.org/10.3390/w12020383
Goeller BC, Febria CM, McKergow LA, Harding JS, Matheson FE, Tanner CC, McIntosh AR. Combining Tools from Edge-of-Field to In-Stream to Attenuate Reactive Nitrogen along Small Agricultural Waterways. Water. 2020; 12(2):383. https://doi.org/10.3390/w12020383
Chicago/Turabian StyleGoeller, Brandon C., Catherine M. Febria, Lucy A. McKergow, Jon S. Harding, Fleur E. Matheson, Chris C. Tanner, and Angus R. McIntosh. 2020. "Combining Tools from Edge-of-Field to In-Stream to Attenuate Reactive Nitrogen along Small Agricultural Waterways" Water 12, no. 2: 383. https://doi.org/10.3390/w12020383