Adsorption of Cu(II) Ions on Adsorbent Materials Obtained from Marine Red Algae Callithamnion corymbosum sp.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of the Adsorbent Materials
2.2. Chemical Reagents
2.3. Adsorption/Desorption Methodology
2.4. Isotherm and Kinetics Modelling of Adsorption Processes
3. Results and Discussion
3.1. Structural Characteristics of Adsorbents
3.2. Influence of Contact Time and Kinetics Modelling
3.3. Influence of Initial Cu(II) Ions Concentration and Isotherm Modelling
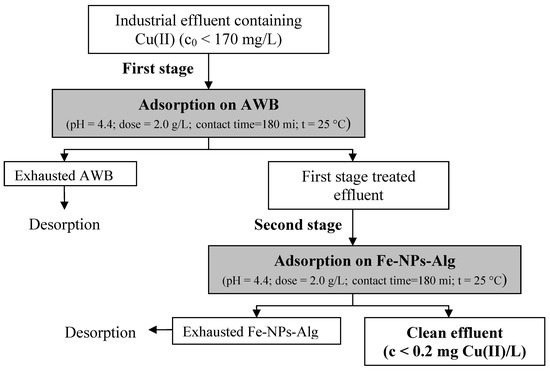
3.4. Practical Applicability
3.5. Desorption and Recovery of Cu(II) Ions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hackbarth, F.V.; Girardi, F.; de Sousa, A.A.U.; Santos, J.C.; Boaventura, R.A.R.; Villar, V.J.P.; Guelli, U.; de Sousa, S.M.A. Ion exchange prediction model for multi-metal system obtained from single-metal systems using the macroalga Pelvetia canaliculata (Phaeophyceae) as a natural cation exchanger. Chem. Eng. J. 2015, 260, 694–705. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B.K. Copper demand, supply, and associated energy use to 2050. Global Environ. Change 2016, 39, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper removal from industrial wastewater: A comprehensive review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Moscatello, N.; Swayambhu, G.; Jones, C.H.; Xu, J.; Dai, N.; Pfeifer, B.A. Continuous removal of copper, magnesium, and nickel from industrial wastewater utilizing the natural product yersiniabactin immobilized within a packed-bed column. Chem. Eng. J. 2018, 343, 173–179. [Google Scholar] [CrossRef]
- Volesky, B. Detoxification of metal-bearing effluents biosorption for the next century. Hydrometallurgy 2015, 59, 2003–2016. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: a review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Tehnol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Aji, B.A.; Yavuz, Y.; Koparal, A.S. Electrocoagulation of heavy metals containing model wastewater using monopolar ion electronedes. Sep. Purif. Technol. 2012, 86, 248–254. [Google Scholar] [CrossRef]
- Blocher, C.; Dorda, J.; Mavrov, V.; Chmiel, H.; Lazaridis, N.K.; Matis, K.A. Hybrid flotation- membrane filtration process for the removal of heavy metals ions from wastewater. Water Res. 2003, 37, 4018–4026. [Google Scholar] [CrossRef]
- Samper, E.; Rodriguez, M.; Rubia, M.A.; Pats, D. Removal pf metal ions at low concentration by micellar – enhanced, ultrafiltration (MEUF), using sodium docecyl sulfate (SDS) and linear alkylbenzene sulfonate(LAS). Sep. Sci. Technol. 2009, 65, 337–342. [Google Scholar]
- Jiang, F.; Yin, S.; Zhang, L.; Peng, J.; Ju, S.; Miller, J.D.; Wang, X. Solvent extraction of Cu(II) from sulfate solutions containing Zn(II) and Fe(III) using an interdigital micromixer. Hydrometallurgy 2018, 177, 116–122. [Google Scholar] [CrossRef]
- Edebali, S.; Pehlivan, E. Evalution of chelate and cation exchange resins to remove copper ions. Powder Tehnol. 2016, 301, 520–525. [Google Scholar] [CrossRef]
- Kang, S.Y.; Lee, J.U.; Moon, S.H.; Kim, K.W. Competitive adsorption characteristics of Co2+, Ni2+, and Cr3+ by IRN-77 cation exchange resin in synthesized wastewater. Chemosphere 2004, 56, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioproc. Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the Art for the Biosorption Process—A Review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef] [Green Version]
- Kratochvil, D.; Volesky, B. Advances in the biosorption of heavy metals. Trends Biotechnol. 1998, 16, 291–300. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015, 20, 1–18. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.H.; Indrawati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef]
- Montazer-Rahmati., M.M.; Rabbani, P.; Abdolali, A.; Keshtkar, A.R. Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. J. Hazard. Mater. 2011, 185, 401–407. [Google Scholar] [CrossRef]
- Romera, E.; Gonzalez, F.; Ballester, A.; Blazquez, M.L.; Munoz, J.A. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modelling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Kyzas, G.Z. Progress in batch biosorption of heavy metals onto algae. J. Molec. Liquids 2015, 209, 77–86. [Google Scholar] [CrossRef]
- Areco, M.M.; Hanela, S.; Duran, J.; dos Santos Afonso, M. Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by dead biomasses of green alga Ulva lactuca and the development of a sustainable matrix for adsorption implementation. J. Hazard. Mater. 2012, 213–214, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Cechinel, M.A.P.; Mayer, D.A.; Pozdniakova, T.A.; Mazur, L.P.; Boaventura, R.A.R.; U. de Souza, A.A.; Guelli U. de Souza, S.M.A.; Vilar, V.J.P. Removal of metal ions from a petrochemical wastewater using brown macro-algae as natural cation-exchangers. Chem. Eng. J. 2016, 286, 1–15. [Google Scholar] [CrossRef]
- Deniz, F.; Ersanli, E.T. A natural macroalgae consortium for biosorption of copper from aqueous solution: Optimization, modeling and design studies. Int. J. Phytoremed. 2018, 20, 362–368. [Google Scholar] [CrossRef]
- Hamed, I.; Ozogul, F.; Ozogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Marine Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [Green Version]
- Fertah, M.; Belfkira, A.; Dahmane, E.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arabian J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef] [Green Version]
- Lucaci, A.R.; Bulgariu, L. Rapid and simple method for the preparation of iron nanoparticles functionalized with alginate and their use as adsorbent. Bull. I.P.Iasi 2019. In press. [Google Scholar]
- Kleinübing, S.J.; da Silva, E.A.; da Silva, M.G.C.; Guibal, E. Equilibrium of Cu(II) and Ni(II) biosorption by marine alga Sargassum filipendula in a dynamic system: Competitiveness and selectivity. Bioresour. Technol. 2011, 102, 4610–4617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, W.; Xu, C.; Qian, G.; Huang, G.; Tang, X.; Lin, B. Adsorption of Cu(II), Zn(II), and Pb(II) from aqueous single and binary metal solutions by regenerated cellulose and sodium alginate chemically modified with polyethyleneimine. RSC Adv. 2018, 8, 18723–18733. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Anu, N.; Nandagopal Giri, M.S.; Selvaraju, N. Relevance of isotherm models in biosorption of pollutants by agricultural by-products. J. Environ. Chem. Eng. 2014, 2, 398–414. [Google Scholar] [CrossRef]
- Deniz, F.; Karabulut, A. Biosorption of heavy metal ions by chemically modified biomass of coastal seaweed community: Studies on phycoremediation system modeling and design. Ecol. 2017, 106, 101–108. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Nguyen, T.V. Applicability of agricultural waste and byproducts for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef]
- NTPA 002/2005. Available online: https://wordpress.com/2015/07/ntpa-002-28-02-2002.pdf (accessed on 7 December 2019).








| Model | Equation | Notations |
|---|---|---|
| Kinetics models | ||
| Pseudo-first order | qe, qt—adsorption capacity at equilibrium and at time t; k1—the rate constant of pseudo-first order kinetic equation; k2—the pseudo-second order rate constant | |
| Pseudo-second order | ||
| Isotherm models | ||
| Langmuir | q—adsorption capacity at equilibrium; qmax—the maximum adsorption capacity; KL—Langmuir constant; KF—Freundlich constant; n—the heterogeneity factor | |
| Freundlich | ||
| Adsorbent | RAB | AWB | Alg | Fe-NPs-Alg |
|---|---|---|---|---|
| qeexp, mg/g | 11.4332 | 10.9632 | 11.3153 | 11.0621 |
| Pseudo-first order kinetic model | ||||
| qe, mg/g | 4.2707 | 7.3824 | 1.0539 | 4.5488 |
| k1, 1/min | 0.0078 | 0.0149 | 0.0125 | 0.0070 |
| R2 | 0.9321 | 0.9265 | 0.7177 | 0.9612 |
| Pseudo-second order kinetic model | ||||
| qe, mg/g | 11.7371 | 11.5207 | 11.3895 | 11.3250 |
| k2, g/mg min | 0.0072 | 0.0086 | 0.0764 | 0.0110 |
| R2 | 0.9954 | 0.9952 | 0.9999 | 0.9977 |
| Adsorbent | RAB | AWB | Alg | Fe-NPs-Alg |
|---|---|---|---|---|
| Langmuir isotherm model | ||||
| qmax, mg/g | 47.62 | 83.33 | 166.66 | 52.63 |
| KL, g/L | 12.81 | 8.82 | 16.92 | 9.54 |
| R2 | 0.935 | 0.988 | 0.966 | 0.846 |
| Freundlich isotherm model | ||||
| 1/n | 0.59 | 1.20 | 0.73 | 0.35 |
| KF, g/L | 7.07 | 1.23 | 3.95 | 30.19 |
| R2 | 0.934 | 0.894 | 0.952 | 0.732 |
| Adsorbent | q, mg/g | qdesorbed, mg/g | D, % |
|---|---|---|---|
| AWB | 31.72 | 30.96 | 97.61 |
| Fe-NPs-Alg | 29.58 | 29.07 | 98.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucaci, A.R.; Bulgariu, D.; Popescu, M.-C.; Bulgariu, L. Adsorption of Cu(II) Ions on Adsorbent Materials Obtained from Marine Red Algae Callithamnion corymbosum sp.. Water 2020, 12, 372. https://doi.org/10.3390/w12020372
Lucaci AR, Bulgariu D, Popescu M-C, Bulgariu L. Adsorption of Cu(II) Ions on Adsorbent Materials Obtained from Marine Red Algae Callithamnion corymbosum sp.. Water. 2020; 12(2):372. https://doi.org/10.3390/w12020372
Chicago/Turabian StyleLucaci, Alina Roxana, Dumitru Bulgariu, Maria-Cristina Popescu, and Laura Bulgariu. 2020. "Adsorption of Cu(II) Ions on Adsorbent Materials Obtained from Marine Red Algae Callithamnion corymbosum sp." Water 12, no. 2: 372. https://doi.org/10.3390/w12020372