-
PDF
- Split View
-
Views
-
Cite
Cite
Morrison O Jusu, Geoffrey Glauser, Jane F Seward, Mohamed Bawoh, Judith Tempel, Michael Friend, Daniel Littlefield, Michael Lahai, Hassan M Jalloh, Amara Bangali Sesay, Amanda F Caulker, Mohamed Samai, Vasavi Thomas, Nicholas Farrell, Marc-Alain Widdowson, Rapid Establishment of a Cold Chain Capacity of –60°C or Colder for the STRIVE Ebola Vaccine Trial During the Ebola Outbreak in Sierra Leone, The Journal of Infectious Diseases, Volume 217, Issue suppl_1, 15 June 2018, Pages S48–S55, https://doi.org/10.1093/infdis/jix336
Close - Share Icon Share
Abstract
In response to the 2014‒2015 Ebola virus disease (Ebola) epidemic in West Africa, researchers accelerated the development of Ebola vaccines. The Centers for Disease Control and Prevention, in collaboration with local partners, sponsored a phase 2/3 trial in Sierra Leone of a single dose (2 × 107 plaque-forming units/mL) of a candidate replication-competent recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein vaccine (rVSVΔG-ZEBOV-GP). Because of its early development status and limited, critical stability data, the vaccine had to be stored at –60°C or colder. Planning for the trial began in late 2014 on an accelerated timeline with significant challenges, including the lack of –60°C or colder vaccine storage, handling, and transport capability in a country with high year-round ambient temperatures. The supply chain needs for vaccine handling from storage through transport, up to the time of administration, were carefully evaluated, and then the supply chain was designed. Critical equipment that was procured, shipped, installed, and qualified to meet an aggressive timeline for launching the trial included ultracold freezers, custom-developed Arktek DF containers for storage at –60°C or colder, insulated containers for storage at 2°C–8°C, and multiple backup power sources at vaccine storage sites. Local personnel were trained in good clinical practices and trial documentation. During 9 April–12 December 2015, the trial staff vaccinated approximately 8000 participants. Five temperature excursions occurred, but the vaccine manufacturer assessed that vaccine quality was not impaired because of improper storage or transport temperatures. Both the infrastructure and the human capacity developed for the cold chain during the trial will continue to be useful as the country maintains preparedness for ring vaccination responses and future clinical research.
ClinicalTrials.gov [NCT02378753] and Pan African Clinical Trials Registry [PACTR201502001037220].
The 2014‒2015 Ebola virus disease (Ebola) epidemic in West Africa stimulated global response efforts and accelerated development of Ebola vaccines and therapeutics [1–3]. By late 2014, phase 1 studies of candidate Ebola vaccines started in the United States, Europe, and Africa [4, 5]. In collaboration with local investigators, international agencies initiated further clinical studies in Sierra Leone, Guinea, and Liberia [6–8]. In Sierra Leone, the Centers for Disease Control and Prevention (CDC) sponsored a phase 2/3 trial—the Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE)—in collaboration with the College of Medicine and Allied Health Sciences, University of Sierra Leone, and the Sierra Leone Ministry of Health and Sanitation (MoHS) [6]. The vaccine tested was a replication-competent recombinant vesicular stomatitis virus–Zaire Ebola virus envelope glycoprotein vaccine (rVSVΔG-ZEBOV-GP).
Sierra Leone is on the Atlantic coast of West Africa, bordered by Guinea to the north and northeast and by Liberia to the south and southeast. The country has sub-Saharan year-round climate conditions (temperature, 22.8°C–31.1°C; relative humidity, 75%), resulting in classification by the World Health Organization (WHO) for Stability Studies as climatic zone IV-B (a hot/humid country, characterized by 2 seasons: one hot and dry and the other wet or rainy). Sierra Leone has one of the world’s lowest gross domestic products and has been undergoing reconstruction to improve its road network and energy distribution following a decade-long civil war that ended in 2002. However, only 12% of the major roads are currently paved, and power interruptions are a daily occurrence. A few major cities, such as Freetown, Makeni, Bo, and Kenema, are connected to the national power grid, which provides intermittent electricity; in some instances, the power supply is rationed to certain parts of these cities on alternate days, so backup power sources are needed. Other areas of the country rely on generators and other sources of power.
The rVSV∆G-ZEBOV-GP candidate vaccine had a stability profile that required storage at –60°C or colder before dilution and at 2°C–8°C after dilution. For STRIVE, we had to transport the vaccine before dilution for up to 120 miles to 2 rural cold chain depots. After dilution, vaccine syringes had to be stored and transported up to 50 miles to 7 vaccination sites across 5 districts; to retain vaccine potency, filled syringes could be stored at 2°C-8°C for up to 12 hours. The equipment, storage and handling sites, and general conditions needed to meet the physical handling requirements for vaccine for this trial were not available in Sierra Leone—a country with no vaccine clinical trial experience. Moreover, STRIVE investigators aimed to launch the trial no more than 3–4 months after the decision to go ahead in late 2014, leaving little time to establish partnerships, regulatory processes, and infrastructure or to recruit and train a staff before beginning the study [6].
In this article, we describe how we established a cold chain system for rVSVΔG-ZEBOV-GP within this short time frame in the face of an unprecedented Ebola outbreak, and we share lessons learned from implementing a clinical trial under these circumstances in a low-resource tropical setting.
STUDY OVERVIEW
STRIVE individually randomly assigned eligible healthcare personnel and frontline Ebola response workers to immediate (within 7 days) or deferred (within 18–24 weeks) vaccination and followed them for 6 months for adverse events or the development of Ebola. For STRIVE, we set up 7 trial enrollment and vaccination sites in 5 districts in Sierra Leone (Figure 1). Enrollment and vaccination began on 9 April 2015, and the last vaccine was administered in the deferred cohort on 12 December 2015. An immunogenicity substudy was also conducted at one site. We obtained ethical and regulatory approvals from the Sierra Leone Ethics and Scientific Review Committee, the CDC Institutional Review Board, the Pharmacy Board of Sierra Leone, and the Food and Drug Administration.
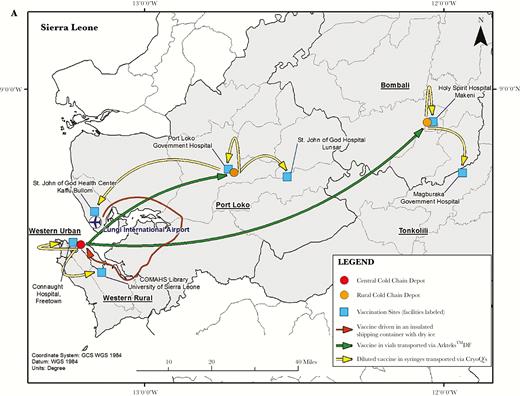
A, The Sierra Leone Trial to Introduce a Vaccine against Ebola (STRIVE) cold chain depots for vaccine storage and transport and study sites for vaccine administration. B, Arktek phase-change material containers and vaccine racks that were modified for use in STRIVE. The Arktek DF container had 3 sets of vaccine carrier racks that filled the space between the phase-change materials. However, the racks were not used to hold vaccines during STRIVE because using them would have meant removing individual vaccine vials from their package carton, which was not feasible without risking the vaccine going out of storage temperature range. Therefore, the vaccines were packed out in Arktek DF containers in their original boxes of 20 vials. C, CryoQ containers packed to show the interior with coolant packs (left), with a close-up view of syringes containing diluted vaccine packed inside (right). A foam pad was then placed on top of the syringes before the CryoQ container was closed.
The manufacturer supplied frozen (temperature, –60°C or colder) rVSVΔG-ZEBOV-GP in 1-mL vials at a concentration of 1 × 108 plaque-forming units (pfu)/mL. The vaccine had to be thawed and diluted with 0.9% normal saline to provide the concentration of 2 × 107 pfu/mL required for administration.
COLD CHAIN FOR STORAGE AND TRANSPORT OF VACCINE
Vaccine Storage
Before STRIVE started, a team from the US Government Biomedical Advanced Research and Development Authority (BARDA), WHO, CDC, and MoHS conducted a needs assessment to evaluate the infrastructure challenges that had to be addressed and the equipment that would have to be purchased to establish the cold chain, not only for the vaccine but also for storing serum specimens collected for the STRIVE immunogenicity substudy.
Because there were no specifically manufactured –60°C freezers on the market, the assessment team recommended that –80°C ultracold freezers be acquired to meet the vaccine storage requirements; this would also allow some leeway (or increase in temperature) in meeting the required –60°C or colder storage temperature (Table 1). Because local procurement was not possible, the freezers were purchased in Europe and Asia, and owing to the urgency of launching the trial, they were shipped via air delivery at significant cost. The team selected 3 locations for housing the freezers: a central depot in Freetown (Sierra Leone’s capital city) and district depots in Port Loko and Makeni (Figure 1A); each was colocated adjacent to Expanded Program on Immunization vaccine storage sites. The freezers required a constant source of electricity to maintain a temperature of –80°C, and all sites required extensive electrical upgrade work and installation of power backups to ensure this. In addition, the freezers had to operate in a controlled temperature environment (range, 20°C–25°C) so their compressors would not fail; ensuring controlled temperatures required building or renovating rooms and installing air conditioners. Because these sites would be used to store potentially life-saving vaccines during the Ebola epidemic, security upgrades were required. A private security company provided 24-hour security for the vaccine at all depots throughout the trial and also supplied fuel to generators as needed. These security personnel also accompanied daily transport of diluted vaccine from the depots to the vaccination sites. Cold chain consultants from Modality Solutions (a cold chain management expert company) accompanied transport of undiluted vaccine vials, which occurred approximately monthly.
Summary of Key Challenges and Resolutions
| Challenge . | Resolution . |
|---|---|
| No –80°C freezers in country | –80°C freezers were procured internationally, shipped, and installed at 3 cold chain depots. |
| No appropriate housing for operating –80°C freezers under necessary ambient temperature conditions | Appropriate building renovations were made at all storage locations, including installation of air conditioning units to maintain ambient temperature <25°C for normal operation of –80°C freezers. |
| No reliable electricity | Multiple power sources were established at each storage site, including further connections to the national grid, batteries that were charged from the national grid, installation of multiple generators, solar panels for lighting, installation of voltage regulators and of both manual and automatic power switches, and use of security personnel for notification of power failure when all else failed (see below). |
| Monitoring electricity supply to the ultracold freezers | Power indicator lights were installed, to denote the status of the power supply to the depots and each of the individual freezers. These were monitored by cold chain staff, and, after hours, they were continuously monitored by onsite security guards with indicator lights at the guard post. If the light went on, indicating a power failure, the security staff alerted cold chain staff immediately. |
| How to transport vaccines across the country at –60°C or colder | Global Good provided Arktek DF containers, passive cooling carriers that were preconditioned with frozen phase-change materials to maintain the required temperatures. |
| How to transport diluted syringes across substantial distances from depots to vaccination sites and monitor temperatures of 2°C–8°C | Passive containers (CRYO-Q containers) were used as vaccine carriers for diluted vaccines, with programmed TempTales to monitor temperature. |
| How to track vaccine dilution batches | Unique dilution numbers were assigned to each batch of 12 syringe doses. |
| How to label each syringe containing a diluted vaccine dose appropriately | Preprinted labels were obtained from the manufacturer, with appropriate identifiers. |
| How to maintain security for candidate Ebola vaccine in an epidemic context | Twenty-four–hour security presence was ensured at all storage sites throughout the vaccine storage period. Security guards also monitored electricity supply to the depots, using lights that activated when power was lost. |
| Challenge . | Resolution . |
|---|---|
| No –80°C freezers in country | –80°C freezers were procured internationally, shipped, and installed at 3 cold chain depots. |
| No appropriate housing for operating –80°C freezers under necessary ambient temperature conditions | Appropriate building renovations were made at all storage locations, including installation of air conditioning units to maintain ambient temperature <25°C for normal operation of –80°C freezers. |
| No reliable electricity | Multiple power sources were established at each storage site, including further connections to the national grid, batteries that were charged from the national grid, installation of multiple generators, solar panels for lighting, installation of voltage regulators and of both manual and automatic power switches, and use of security personnel for notification of power failure when all else failed (see below). |
| Monitoring electricity supply to the ultracold freezers | Power indicator lights were installed, to denote the status of the power supply to the depots and each of the individual freezers. These were monitored by cold chain staff, and, after hours, they were continuously monitored by onsite security guards with indicator lights at the guard post. If the light went on, indicating a power failure, the security staff alerted cold chain staff immediately. |
| How to transport vaccines across the country at –60°C or colder | Global Good provided Arktek DF containers, passive cooling carriers that were preconditioned with frozen phase-change materials to maintain the required temperatures. |
| How to transport diluted syringes across substantial distances from depots to vaccination sites and monitor temperatures of 2°C–8°C | Passive containers (CRYO-Q containers) were used as vaccine carriers for diluted vaccines, with programmed TempTales to monitor temperature. |
| How to track vaccine dilution batches | Unique dilution numbers were assigned to each batch of 12 syringe doses. |
| How to label each syringe containing a diluted vaccine dose appropriately | Preprinted labels were obtained from the manufacturer, with appropriate identifiers. |
| How to maintain security for candidate Ebola vaccine in an epidemic context | Twenty-four–hour security presence was ensured at all storage sites throughout the vaccine storage period. Security guards also monitored electricity supply to the depots, using lights that activated when power was lost. |
Summary of Key Challenges and Resolutions
| Challenge . | Resolution . |
|---|---|
| No –80°C freezers in country | –80°C freezers were procured internationally, shipped, and installed at 3 cold chain depots. |
| No appropriate housing for operating –80°C freezers under necessary ambient temperature conditions | Appropriate building renovations were made at all storage locations, including installation of air conditioning units to maintain ambient temperature <25°C for normal operation of –80°C freezers. |
| No reliable electricity | Multiple power sources were established at each storage site, including further connections to the national grid, batteries that were charged from the national grid, installation of multiple generators, solar panels for lighting, installation of voltage regulators and of both manual and automatic power switches, and use of security personnel for notification of power failure when all else failed (see below). |
| Monitoring electricity supply to the ultracold freezers | Power indicator lights were installed, to denote the status of the power supply to the depots and each of the individual freezers. These were monitored by cold chain staff, and, after hours, they were continuously monitored by onsite security guards with indicator lights at the guard post. If the light went on, indicating a power failure, the security staff alerted cold chain staff immediately. |
| How to transport vaccines across the country at –60°C or colder | Global Good provided Arktek DF containers, passive cooling carriers that were preconditioned with frozen phase-change materials to maintain the required temperatures. |
| How to transport diluted syringes across substantial distances from depots to vaccination sites and monitor temperatures of 2°C–8°C | Passive containers (CRYO-Q containers) were used as vaccine carriers for diluted vaccines, with programmed TempTales to monitor temperature. |
| How to track vaccine dilution batches | Unique dilution numbers were assigned to each batch of 12 syringe doses. |
| How to label each syringe containing a diluted vaccine dose appropriately | Preprinted labels were obtained from the manufacturer, with appropriate identifiers. |
| How to maintain security for candidate Ebola vaccine in an epidemic context | Twenty-four–hour security presence was ensured at all storage sites throughout the vaccine storage period. Security guards also monitored electricity supply to the depots, using lights that activated when power was lost. |
| Challenge . | Resolution . |
|---|---|
| No –80°C freezers in country | –80°C freezers were procured internationally, shipped, and installed at 3 cold chain depots. |
| No appropriate housing for operating –80°C freezers under necessary ambient temperature conditions | Appropriate building renovations were made at all storage locations, including installation of air conditioning units to maintain ambient temperature <25°C for normal operation of –80°C freezers. |
| No reliable electricity | Multiple power sources were established at each storage site, including further connections to the national grid, batteries that were charged from the national grid, installation of multiple generators, solar panels for lighting, installation of voltage regulators and of both manual and automatic power switches, and use of security personnel for notification of power failure when all else failed (see below). |
| Monitoring electricity supply to the ultracold freezers | Power indicator lights were installed, to denote the status of the power supply to the depots and each of the individual freezers. These were monitored by cold chain staff, and, after hours, they were continuously monitored by onsite security guards with indicator lights at the guard post. If the light went on, indicating a power failure, the security staff alerted cold chain staff immediately. |
| How to transport vaccines across the country at –60°C or colder | Global Good provided Arktek DF containers, passive cooling carriers that were preconditioned with frozen phase-change materials to maintain the required temperatures. |
| How to transport diluted syringes across substantial distances from depots to vaccination sites and monitor temperatures of 2°C–8°C | Passive containers (CRYO-Q containers) were used as vaccine carriers for diluted vaccines, with programmed TempTales to monitor temperature. |
| How to track vaccine dilution batches | Unique dilution numbers were assigned to each batch of 12 syringe doses. |
| How to label each syringe containing a diluted vaccine dose appropriately | Preprinted labels were obtained from the manufacturer, with appropriate identifiers. |
| How to maintain security for candidate Ebola vaccine in an epidemic context | Twenty-four–hour security presence was ensured at all storage sites throughout the vaccine storage period. Security guards also monitored electricity supply to the depots, using lights that activated when power was lost. |
Global Good, as part of Intellectual Ventures, procured 8 ultracold freezers (temperature, –80°C) for use in STRIVE; 4 freezers with a capacity of 500–1000 L were placed at the central depot in Freetown, and 2 freezers with a capacity of 500-L were placed at each of the district depots (Figure 1A). Supporting electrical panels were upgraded to power a minimum of two –80°C freezers and a refrigerator at each depot. One freezer was used for storing vaccine and the other for preconditioning phase-change materials (PCMs) for portable passive vaccine storage devices (Arktek DF; developed by Intellectual Ventures/Global Good [Bellevue, WA] for storage and transport of vaccines for the Expanded Program on Immunizations and modified for the purpose of this clinical trial), and the refrigerator was used to cool the 0.9% normal saline vaccine diluent and to condition coolant packs used for transport of the diluted-vaccine syringes from the depot to the vaccination sites. STRIVE cold chain staff stored the PCM coolant packs that were used in the Arktek DF containers in the freezers used for vaccine storage. This assisted in stabilizing interior freezer temperatures and provided short-term capability to reduce temperature fluctuations due to power failures.
Monitoring the power supply 24 hours a day and providing backup sources of electricity (Table 1) were required for ensuring a reliable electricity supply for the cold chain equipment, performing daily cold chain activities, and maintaining the low freezer temperatures. Two depot facilities were connected to the national power grid as their primary source of electricity; the third, at Port Loko, which is not connected to the national grid, relied on a generator. The final power configurations at each of the depots were designed to withstand frequent primary power outages by providing at least 2 backup alternative power sources; these included generators and batteries. Solar panels generated power to run lighting at the Port Loko cold chain site. Additional precautions to overcome power supply irregularities included the following, some of which were implemented after the trial started as cold chain staff gained experience with frequent power outages. First, voltage regulators were installed, to minimize the impact of power surges. Second, automatic power switches were installed, to provide smooth and rapid transfer of power from grid to generator or battery backup and vice versa when the power supply was interrupted and again later when it was restored. Initially, STRIVE used manual power switches for transfer of power to backup systems. Third, power indicator lights were installed, to denote the status of the power supply to the depots and each of the individual freezers. These were monitored by cold chain staff, and, after hours, they were continuously monitored by onsite security guards with indicator lights at the guard post. If the light went off, indicating a power failure, the security staff would alert cold chain staff immediately.
Even with multiple sources of electricity and backup plans in place, power interruptions to the freezers occurred frequently, for a variety of reasons, including failure of the grid, mechanical or electrical faults (eg, malfunction of an automatic switch), and human factors (eg, a guard not adding fuel to the generator or leaving his watch during the night). Moreover, 2 freezers failed before or during the trial and could not be fixed in Sierra Leone; therefore, new freezers or alternative solutions for temporary vaccine storage had to be provided.
We also used the central vaccine storage site for temporary storage of serum samples collected for the immunogenicity substudy, because it had the freezers and trained, experienced staff needed for safe storage of these samples. We stored the samples in a separate freezer from the study vaccine or other pharmaceutical products and later transported them to US laboratories for testing with a validated assay.
Vaccine Transport
The STRIVE vaccine was shipped in polyurethane-insulated containers on pelletized dry ice via air cargo from the United States to Lungi International Airport in Sierra Leone. After customs clearance, the vaccine was transferred by vehicle to the central depot (3.5 hours by road). Four vaccine shipments occurred during the trial. Because many flights to Sierra Leone were cancelled during the epidemic, the first 3 shipments were complicated, with itineraries changing before and during shipment, delays occurring because flights were cancelled, and because the vaccine, as a cargo shipment, was lower priority than passenger luggage for loading onto planes. The shipping company monitored shipment temperatures, and dry ice had to be replenished during transit delays. Shipment durations ranged from 3 to 10 days.
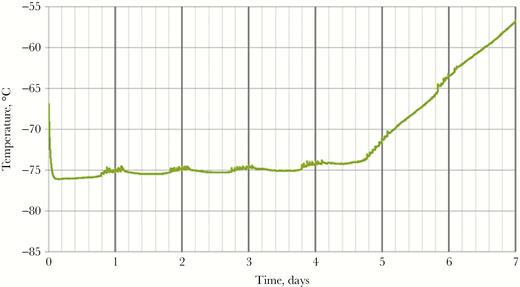
The central depot supplied vaccine to the 2 district depots approximately monthly. Without any local source of dry ice, the assessment team recommended acquiring Arktek containers to fill the critical need for transporting the vaccines at –60°C or colder from the central Freetown depot to the district depots (Figure 1 and Table 1). The Arktek container is a double-walled stainless steel vacuum-assisted insulated drum that was developed for storage of Expanded Program on Immunizations vaccines at 2°C–8°C for up to 1 month. The device was modified (and designated as “Arktek DF”; hereafter referred to as “Arktek”) to handle –80°C (as with the ultracold freezers, to allow some leeway in maintaining the required vaccine storage temperature of –60°C or colder) for the Ebola vaccine trials, with a potential payload of approximately 200 vials of vaccine (Figure 1B) [9]. This was accomplished by substituting a combination of phase-change proprietary denatured alcohols that were titrated to freeze and thaw at –80°C for the water/ice contained in the 8 crescent-shaped plastic or metal containers inside the 35-pound drum. The filled phase-change containers were preconditioned in the ultracold freezers at –85.5°C to reach the solid crystallization threshold. Once this temperature was reached, the liquids changed to solids, and the interior temperature was efficiently maintained in the ultracold freezers. When needed, the PCMs were loaded into the Arktek containers. Because STRIVE leaders were concerned that repeated opening of an Arktek container in sub-Saharan temperatures over several days would compromise its ability to maintain a temperature of –60°C or colder, Intellectual Ventures conducted laboratory qualification tests in which the Arktek containers were opened 8 times a day (to simulate removal of vaccine) at ambient temperatures of 32°C and 42°C. These tests showed that temperatures colder than –60°C could be maintained for >6 days in those conditions [9] (Figure 2). Eight Arktek containers were provided for STRIVE; 5–6 were used regularly, and the remaining 2–3 were on standby. Under field conditions, temperatures in the Arktek containers remained in the range of –74°C to –70°C for about 5 days, well below the required temperature of –60°C or colder. Each depot always had at least 1 Arktek container that was preconditioned to use to transfer vaccine between depots and for storage in the event of freezer failure. At the Port Loko depot, preconditioned Arktek containers were rotated for use, to permanently store the vaccine owing to failure of one of the ultracold freezers.
Temperature of the vaccine compartment of the Arktek DF container during laboratory qualification testing. During the testing, the Arktek DF container was opened 8 times a day, 1 hour apart, and the temperature in the vaccine compartment was continuously monitored. The phase-change material (PCM) appears to have fully melted by 5 days, but since it took approximately 1.5 days after PCM melting for the system to warm to –60°C, the hold time of the –78° PCMs was just under 6.5 days.
Providing Vaccine to Vaccination Sites
Because the manufacturer provided the vaccine in 1-mL vials at a concentration of 1 × 108 pfu/mL, it had to be diluted to the required concentration of 2 × 107 pfu/mL before administration. Because no traditional pharmacies were located at study locations, the cold chain sites served that purpose. The Freetown site functioned as the central study pharmacy, and trained pharmacists handled study vaccine dispensation at all the sites. We developed facilities to allow for vaccine dilution at all 3 depots. Following standard operating procedures and sterile transfer techniques, trained pharmacy staff thawed the vaccine at ambient temperatures of <25°C and then diluted 2.7 mL from three 1-mL vials of vaccine into a vial containing 10.8 mL of 0.9% normal saline. This resulted in the required 5-fold vaccine dilution and sufficient vaccine in the diluent vial to draw up (allowing for wastage) 12 syringes, each with 1 mL of the correct vaccine dose. The use of vial adapters to draw up vaccine into syringes from the diluent vial helped promote sterile conditions and significantly streamlined the dilution process with Leur-Lok connections. The manufacturer provided preprinted syringe labels with appropriate identifiers, and the syringes were maintained at a temperature of 2°C–8°C for up to 12 hours before administration to participants.
Passive insulated reusable containers (CryoQ; Lisaline Lifescience Technologies, Thane, India), were used to transport vaccine from depots to vaccination sites at a temperature of 2°C–8°C. The WHO provided 15 of these containers in Sierra Leone on very short notice, and Intellectual Ventures/Global Good flew in another 20 containers later. Engineers from BARDA and Modality Solutions tested and qualified the CryoQ containers to ensure they could maintain a temperature of 2°C–8°C for 12 hours despite repeated opening to retrieve vaccine syringes. To achieve and maintain this temperature range, the CryoQ containers had to be preconditioned with 1 frozen gel pack and 3 refrigerated ice packs. The CryoQ container was then closed with fitted-in foam packs to keep syringes secure and the enclosed temperature monitoring device from touching the inside walls of the CryoQ container (Figure 1C).
To minimize wastage and plan daily vaccine dilutions for participants randomly assigned to the immediate group, STRIVE cold chain depot staff maintained regular communication with enrollment and vaccination sites regarding the expected and actual number of participants. Participants randomly assigned to the immediate vaccination group were not given appointments, because of the unpredictability of their work schedules during the Ebola epidemic, so vaccinators did not know in advance the exact number of participants who would be vaccinated each day. Therefore, based on estimates from previous days, pharmacists diluted and provided syringes to start vaccination at each site and then communicated with the site managers during the day to assess whether additional vaccines would be required. Because each dilution required 3 vaccine vials to produce 12 doses, such planning and ongoing communication were important for reducing vaccine wastage.
In contrast, for the participants in the deferred vaccination group, schedulers set up visits in advance and made telephone calls reminding participants to come for vaccination on their appointed days. This contributed to a reduction in wastage of vaccine doses across all the sites, from 32% during immediate vaccination to 22% during deferred vaccination (Table 2).
Vaccine Doses Prepared and Wastage per Vaccination Site, by Study Phase
| Site . | Immediate Vaccination . | Deferred Vaccination . | ||||
|---|---|---|---|---|---|---|
| Doses Prepared, No. . | Doses Administered, No. . | Doses Wasted, % . | Doses Prepared, No. . | Doses Administered, No. . | Doses Wasted, % . | |
| Connaught Hospital, Freetown | 2290 | 1553 | 32 | 1736 | 1391 | 20 |
| COMAHS Library | 1174 | 902 | 23 | 1060 | 852 | 20 |
| Port Loko Government Hospital | 626 | 423 | 32 | 516 | 399 | 23 |
| St. John of God Hospital, Lunsar | 319 | 194 | 39 | 288 | 170 | 41 |
| St. John of God Health Center, Lungi | 312 | 150 | 52 | 216 | 143 | 34 |
| Holy Spirit Hospital, Makeni | 870 | 595 | 32 | 744 | 546 | 27 |
| Magburaka District Hospital | 528 | 366 | 31 | 384 | 338 | 12 |
| Total | 6119 | 4183 | 32 | 4944 | 3839 | 22 |
| Site . | Immediate Vaccination . | Deferred Vaccination . | ||||
|---|---|---|---|---|---|---|
| Doses Prepared, No. . | Doses Administered, No. . | Doses Wasted, % . | Doses Prepared, No. . | Doses Administered, No. . | Doses Wasted, % . | |
| Connaught Hospital, Freetown | 2290 | 1553 | 32 | 1736 | 1391 | 20 |
| COMAHS Library | 1174 | 902 | 23 | 1060 | 852 | 20 |
| Port Loko Government Hospital | 626 | 423 | 32 | 516 | 399 | 23 |
| St. John of God Hospital, Lunsar | 319 | 194 | 39 | 288 | 170 | 41 |
| St. John of God Health Center, Lungi | 312 | 150 | 52 | 216 | 143 | 34 |
| Holy Spirit Hospital, Makeni | 870 | 595 | 32 | 744 | 546 | 27 |
| Magburaka District Hospital | 528 | 366 | 31 | 384 | 338 | 12 |
| Total | 6119 | 4183 | 32 | 4944 | 3839 | 22 |
Vaccine Doses Prepared and Wastage per Vaccination Site, by Study Phase
| Site . | Immediate Vaccination . | Deferred Vaccination . | ||||
|---|---|---|---|---|---|---|
| Doses Prepared, No. . | Doses Administered, No. . | Doses Wasted, % . | Doses Prepared, No. . | Doses Administered, No. . | Doses Wasted, % . | |
| Connaught Hospital, Freetown | 2290 | 1553 | 32 | 1736 | 1391 | 20 |
| COMAHS Library | 1174 | 902 | 23 | 1060 | 852 | 20 |
| Port Loko Government Hospital | 626 | 423 | 32 | 516 | 399 | 23 |
| St. John of God Hospital, Lunsar | 319 | 194 | 39 | 288 | 170 | 41 |
| St. John of God Health Center, Lungi | 312 | 150 | 52 | 216 | 143 | 34 |
| Holy Spirit Hospital, Makeni | 870 | 595 | 32 | 744 | 546 | 27 |
| Magburaka District Hospital | 528 | 366 | 31 | 384 | 338 | 12 |
| Total | 6119 | 4183 | 32 | 4944 | 3839 | 22 |
| Site . | Immediate Vaccination . | Deferred Vaccination . | ||||
|---|---|---|---|---|---|---|
| Doses Prepared, No. . | Doses Administered, No. . | Doses Wasted, % . | Doses Prepared, No. . | Doses Administered, No. . | Doses Wasted, % . | |
| Connaught Hospital, Freetown | 2290 | 1553 | 32 | 1736 | 1391 | 20 |
| COMAHS Library | 1174 | 902 | 23 | 1060 | 852 | 20 |
| Port Loko Government Hospital | 626 | 423 | 32 | 516 | 399 | 23 |
| St. John of God Hospital, Lunsar | 319 | 194 | 39 | 288 | 170 | 41 |
| St. John of God Health Center, Lungi | 312 | 150 | 52 | 216 | 143 | 34 |
| Holy Spirit Hospital, Makeni | 870 | 595 | 32 | 744 | 546 | 27 |
| Magburaka District Hospital | 528 | 366 | 31 | 384 | 338 | 12 |
| Total | 6119 | 4183 | 32 | 4944 | 3839 | 22 |
QUALITY CONTROL AND MONITORING SYSTEMS
Temperature Monitoring
We put systems in place to ensure that cold chain standards and requirements were maintained and recorded according to good clinical practice, to monitor rVSVΔG-ZEBOV-GP at 2 temperatures, –60°C or colder before dilution and 2°C–8°C after dilution. If temperature excursions occurred outside these specifications, we immediately quarantined the vaccine, investigated the excursion, and reported the excursion to quality experts at NewLink Genetics (the vaccine manufacturer; the vaccine was later licensed to Merck). These experts reported the final disposition of the vaccine, based on the temperature excursion profile and stability data.
We used 2 types of devices to monitor vaccine at temperatures of –60°C or colder: the TempTale4 device (Sensitech; Beverly, MA) and the HOBO device (Onset Computer; Bourne, MA). During vaccine shipment from the United States to Sierra Leone, a TempTale4 device recorded the temperature in transit. Upon the shipment container’s arrival at the Freetown central depot, we downloaded the temperature profile from the monitoring device to a computer and sent it to the vaccine manufacturer for review. The storage freezers were set at –85.5°C but were programmed to alarm at temperatures warmer than –70°C. We used 2 independent methods to monitor freezer temperatures: (1) twice-daily manual recording of temperatures from built-in temperature displays on the freezers and (2) continuous electronic temperature monitoring via a HOBO device, which had thermocouple probes that monitor the interior temperatures of the equipment to which it is connected; their outputs can be read on the HOBO display. We programmed the HOBO device to display a visual indicator (alarm bell icon) when freezer temperatures went outside limits. When depot staff saw the alarm indicator, they immediately noted a deviation and initiated an investigation. HOBO devices were also fitted for each Arktek container. Cold chain staff also recorded storage depots’ ambient temperatures twice daily.
To monitor the temperature of diluted vaccine in syringes at a temperature of 2°C–8°C, we initialized programmable TempTale4 devices and included one in each CryoQ pack sent to vaccination sites. At days’ end, we downloaded, saved, printed, and attached the recorded temperature data from the TempTale4 device to the other records of the respective diluted vaccine set.
During STRIVE, 5 temperature excursions occurred. Three of these were associated with frozen concentrated vaccine. Two of the excursions were a result of catastrophic failure of key electrical equipment. The first of these was a short circuit in the junction box feeding power to the central depot cold chain storage facility. The second was a catastrophic short circuit in a plug that contributed to overload of the battery/automatic transfer system at the central depot. This was the worst excursion, lasting 5 hours 50 minutes, with the freezer temperature reaching –53°C. The third excursion occurred at Port Loko, when the main generator went offline and the guard assigned to start up the backup generator was absent from his post. Two excursions were associated with the diluted vaccine. Both resulted from improper preconditioning of the refrigerated gel packs used in the CryoQ packs. In one instance, the gel ices were too warm, resulting in higher temperatures (up to 18°C) for the vaccine for as long as 3 hours 5 minutes. In the second instance, the refrigerated gel packs were too cold, allowing the vaccine temperature to drop to 0°C, although it did not freeze. For all excursions, the vaccine manufacturer determined that the duration and magnitude were small enough that the vaccine quality was not adversely affected.
Electronic Alert Systems
Implementing STRIVE, we learned that having a system for alerting cold chain staff members about power failures and potential temperature excursions was essential but also challenging, owing to unreliable power, Internet, and cellular services. Initially, we installed temperature signaling devices in each –80°C freezer that would send a text message to cold chain staff’s cell phones when an alarm-associated temperature of –70°C was reached. However, this system proved to be unreliable because of inconsistent cellular connectivity at the central depot, and we replaced it with a WiFi Internet–based system. This system also was eventually abandoned owing to unreliable connectivity. Ultimately, we relied on continuous monitoring by security guards, who notified the cold chain staff via text message or telephone call when they discovered an alarm light triggered by power failure.
Inventory Management
Trained study pharmacists managed vaccine dispensation at each cold chain site. Inventory management included monitoring each of the following: storing frozen vaccine at the depots, transporting vaccine between depots, diluting and transporting vaccines in syringe doses to vaccination sites, and maintaining ancillary supplies. Unused syringes were returned to the depot at the end of each day for incineration, along with any damaged or rejected syringes, and wastage was closely monitored. We documented vaccine inventory and chain of custody in compliance with STRIVE standard operating procedures. We also strictly accounted for manufacturer-supplied syringe labels, per good clinical practice.
Because needed materials were not available locally, we monitored and replenished satisfactory inventories of ancillary supplies (ie, syringes, syringe caps, needles, and vial adapters) through international purchasing arrangements with suppliers in Europe, both before and during the trial.
HUMAN RESOURCE CAPACITY AND MANAGEMENT OF COLD CHAIN
Fifteen staff members were directly involved in STRIVE cold chain activities throughout the trial; 3 were rotating international cold chain experts from BARDA and Modality Solutions, and 12 were national staff members (8 pharmacists and 4 cold chain officers). The latter 12 staff members were divided among the 3 depots. The local staff received role-specific trainings according to the protocol and standard operating procedures for cold chain activities needed for STRIVE, as well as general training on International Conference on Harmonization guidelines on good clinical practice, human subjects protection, and good documentation practice. International cold chain experts provided the initial and refresher trainings, supervision, and onsite oversight for cold chain activities throughout the trial. After vaccination ended, local staff managed oversight of storage of the remaining vaccine vials, with remote backup from international consultants.
SUMMARY AND CONCLUSIONS
In just 3 months, STRIVE staff successfully established and maintained a cold chain for storing the candidate Ebola vaccine at a temperature of –60°C or colder for the phase 2/3 trial of rVSV∆G-ZEBOV-GP vaccine in Sierra Leone. The staff successfully administered about 8000 doses of vaccine, with 28% of doses wasted overall at the vaccination sites and <0.1% of doses rejected or damaged. Challenges encountered and overcome included poor infrastructure for cold chain facilities, lack of highly specialized equipment needed for storing vaccine at extremely low temperatures, high ambient temperatures, 24-hour temperature monitoring for vaccine, interruptions and surges in the power supply, and freezer equipment failure. The modified Arktek devices, capable of maintaining extremely low temperatures without power, proved invaluable and reliable for transporting the vaccines and as backup vaccine storage at all 3 storage sites during temporary periods of power loss or freezer malfunction. Overall, despite frequent interruptions of the power supply to the ultracold freezers, significant temperature excursions (warmer than –60°C) for undiluted vaccine occurred only 3 times during the trial, and excursions outside the temperature range of 2°C–8°C for diluted vaccine occurred only twice. In all these instances, product stability data confirmed that the excursion had no effect on product quality, and no vaccine doses had to be discarded. This was possible because of the excellent monitoring processes and rapid emergency response to deviations that we put in place for the trial.
The Guinea Ebola ça Suffit trial of rVSV∆G-ZEBOV-GP vaccine reported 100% vaccine efficacy, paving the way for use of this vaccine in response to confirmed Ebola cases [10–13]. One lasting legacy of STRIVE is that the cold chain infrastructure, specialized equipment, and local staff expertise are now available in Sierra Leone for handling, according to good clinical practice, storage at a temperature of –60°C or colder, dilution of vaccine, and transport of vaccine at a temperature of 2°C–8°C, under the challenging conditions frequently encountered there. This will facilitate use of the rVSV∆G-ZEBOV-GP vaccine under emergency access protocols in ring response to future Ebola cases both in Sierra Leone and elsewhere [14]. The central depot facilities also were able to store several thousand immunogenicity specimens that were collected during STRIVE. Building local human resource capacity for conducting clinical trials and, in particular, for managing the cold chain means that these newly acquired knowledge and skills can be used to benefit the country in future clinical trials and routine national immunization activities.
Notes
Acknowledgments. We thank Mark Feinberg and Swati Gupta from Merck, for providing vaccine and technical support; Tom Monath and Jay Ramsey from NewLink Genetics, for advice on dilution, vaccine management during temperature excursions, vaccine shipping, and consumables; Simone Zipursky, Souleymane Kone, Theo Grace, and Argimiro (Miro) Garcia Montes from the WHO, for their technical assistance in advising on cold chain facilities and equipment; Sandra Pizzaro from the US Embassy in Freetown, for assistance with packages, notifications, and general STRIVE support; the staff of eHealth, for support in coordinating and supporting renovation and sourcing supplies; and Joerg Ropel, Chief of Operations, for generous assistance and expertise, and other staff from the German Federal Agency for Technical Relief.
Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC), the Biomedical Advanced Research and Development Authority, and the National Institutes of Health, with additional support from the CDC Foundation.
Supplement sponsorship. This work is part of a supplement sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. M. F. has patents (8 377 030 and 8 887 944) licensed to Aucma Global Medical. D. L. reports that he was contracted to the work for the study. All other authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Present affiliation. Partnership for Influenza Vaccine Introduction, Center for Vaccine Equity, Task Force for Global Health, Atlanta, Georgia (J. F. S.).
Retired.