-
PDF
- Split View
-
Views
-
Cite
Cite
Noah C Peeri, Nistha Shrestha, Md Siddikur Rahman, Rafdzah Zaki, Zhengqi Tan, Saana Bibi, Mahdi Baghbanzadeh, Nasrin Aghamohammadi, Wenyi Zhang, Ubydul Haque, The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned?, International Journal of Epidemiology, Volume 49, Issue 3, June 2020, Pages 717–726, https://doi.org/10.1093/ije/dyaa033
Close - Share Icon Share
Abstract
To provide an overview of the three major deadly coronaviruses and identify areas for improvement of future preparedness plans, as well as provide a critical assessment of the risk factors and actionable items for stopping their spread, utilizing lessons learned from the first two deadly coronavirus outbreaks, as well as initial reports from the current novel coronavirus (COVID-19) epidemic in Wuhan, China.
Utilizing the Centers for Disease Control and Prevention (CDC, USA) website, and a comprehensive review of PubMed literature, we obtained information regarding clinical signs and symptoms, treatment and diagnosis, transmission methods, protection methods and risk factors for Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS) and COVID-19. Comparisons between the viruses were made.
Inadequate risk assessment regarding the urgency of the situation, and limited reporting on the virus within China has, in part, led to the rapid spread of COVID-19 throughout mainland China and into proximal and distant countries. Compared with SARS and MERS, COVID-19 has spread more rapidly, due in part to increased globalization and the focus of the epidemic. Wuhan, China is a large hub connecting the North, South, East and West of China via railways and a major international airport. The availability of connecting flights, the timing of the outbreak during the Chinese (Lunar) New Year, and the massive rail transit hub located in Wuhan has enabled the virus to perforate throughout China, and eventually, globally.
We conclude that we did not learn from the two prior epidemics of coronavirus and were ill-prepared to deal with the challenges the COVID-19 epidemic has posed. Future research should attempt to address the uses and implications of internet of things (IoT) technologies for mapping the spread of infection.
Inadequate risk assessment by the Chinese government hampered efforts to contain the virus.
The current novel coronavirus (COVID-19) has surpassed severe acute respiratory syndrome (SARS) in the number of cases and deaths from the disease.
Closure of the live-animal markets in China may decrease the likelihood of another zoonotic outbreak occurring.
Human-to-human transmission has been confirmed, and although several measures have been taken to mitigate the virus’ spread, travel to impacted regions should be avoided if possible.
Introduction
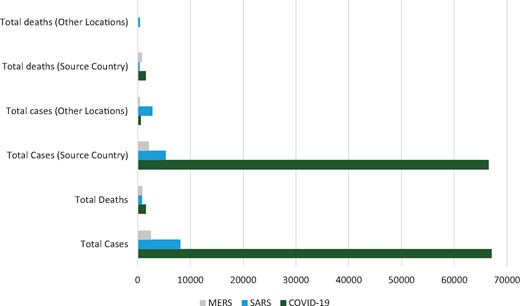
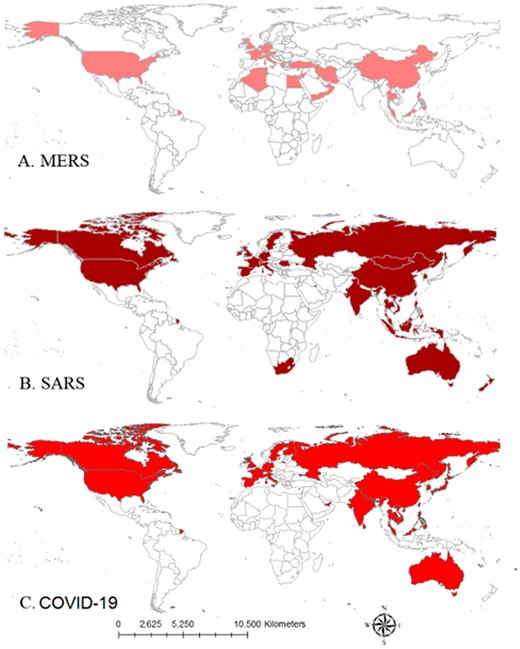
The novel coronavirus (COVID-19) was first identified in Wuhan, China, in December 2019 among a cluster of patients that presented with an unidentified form of viral pneumonia with shared history of visiting the Huanan seafood market.1 Patients were assessed for viral pneumonia through the ascertainment and testing of bronchoalveolar-lavage fluid utilizing whole genome sequencing, cell cultures and polymerase chain reaction (PCR). The virus was isolated from biologic samples and identified as genus betacoronavirus, placing it alongside severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS).1 At the time of writing, the number of persons infected by the virus has now surpassed 67 091 and Chinese authorities have reported 1527 deaths from the virus, most in Hubei, the provincial epicenter of the outbreak.2 Over 25 countries have confirmed cases to date, including countries from Asia, Europe, North America and the Middle East (see Figure 1).2 The virus spread internationally within 1 month of the first identification, and can be transmitted via close human-to-human contact.3 The World Health Organization (WHO) declared COVID-19 a Public Health Emergency of International Concern as of 1 February 2020.
Another beta-coronavirus was first identified in Southern China (Guangdong province) in November 2002. The WHO did not receive an update from the Chinese government until the end of March, with 792 cases and 31 deaths reported. The lack of transparency of the Chinese health ministry has been cited as one of the largest contributors to the spread of the virus globally.4 At the end of the epidemic, China reported >8,000 cases of the disease and 774 deaths, and a case-fatality rate of 7%.5 The reservoir host of the disease was thought to be the Asian civet cat (Paguma larvata). The foci of transmission from host to human were thought to be the open markets, much like the COVID-19 outbreak currently ongoing.5 The SARS global outbreak was contained in July 2003 and since 2004 there have not been any known cases of SARS reported.6
After the emergence of SARS, MERS was the second coronavirus resulting in a major global public health crisis. It first emerged in 2012 in Saudi Arabia when a 60 year-old man presented with severe pneumonia.7 An outbreak of the virus did not occur until 2 years later, in 2014, with a total number of identified cases of 662 and a 32.97% case-fatality rate.8 From 2014 to 2016, 1364 cases were observed in Saudi Arabia. A total of 27 countries were affected by MERS during the outbreaks spanning Europe, Asia, the Middle East and North America. Cases that were identified outside of the Middle East, including the outbreak in South Korea in which 186 individuals were infected as a result of a super spreader, were transplanted individuals that had previously been infected in the Middle East.9 Since 2012, 2494 laboratory confirmed cases of MERS have been reported, and 858 associated deaths have occurred (34.4% case-fatality ratio).8,10
The objectives of our study are to provide an overview of the three major deadly coronaviruses and identify areas for improvement of future preparedness plans, as well as provide a critical assessment of the risk factors and actionable items for stopping their spread, utilizing lessons learned from the first two deadly coronavirus outbreaks, as well as initial reports from the current COVID-19 epidemic in Wuhan, China. Although the epidemic is still ongoing, initial lessons from its spread can help inform public health officials and medical practitioners in efforts to combat its progression.
Methods
Utilizing the Centers for Disease Control and Prevention (CDC, USA) website, and a comprehensive review of PubMed literature, we obtained information regarding clinical signs and symptoms, treatment and diagnosis, transmission methods, protection methods and risk factors for MERS, SARS and COVID-19. Additionally, the Chinese Center for Disease Control and Prevention (CCDC) was accessed for up-to-date information on COVID-19. Furthermore, verified news articles were also of interest in obtaining up-to-date case and fatality numbers on COVID-19. The Johns Hopkins University website was also utilized to access maps and spatio-temporal information regarding the virus.2 SARS and MERS data were compiled from the WHO’s latest situation report for creation of graphs and maps to compare the spatial distribution of the three coronaviruses.
Patient and public involvement
Patients and the public were not involved in this research.
Results
Diagnosis and treatment
With respect to COVID-19, diagnosis was conducted initially by assessing clinical characteristics of the presenting patient, chest imaging and the ruling out of common bacterial and viral pneumonia. Once common bacterial and viral pathogens were ruled out, lower and upper respiratory tract specimens were obtained for cell culture and deep sequencing analysis. These specimens indicated a novel coronavirus initially known as ‘2019-nCoV’.3 PCR, using the ‘RespiFinderSmart22kit’ (PathoFinder BV) real-time reverse transcription PCR (RT-PCR) assay, was used to detect viral RNA by targeting a consensus RNA dependent RNA polymerase region of pan β-CoV.3 A diagnostic test was developed soon after viral isolation. Treatment in some hospitals involves prophylactic antibiotics to prevent secondary infection.3 To date, no antiviral agent has been proven effective against COVID-19. Initial reports showed that oseltamivir was given to 93% of patients (orally administered 75 mg 2x/day) in combination with antibiotics.3 Patients experiencing severe illness (22%) were given corticosteroids (40–120 mg/day) to reduce lung inflammation due to high levels of cytokines caused by the virus, as part of a combined regimen for cases that were community-acquired and diagnosed at the designated hospital.3 Since the combination of lopinavir and ritonavir was already available in the local hospital, a randomized controlled trial was initiated quickly to assess the efficacy and safety of combined use of lopinavir and ritonavir in patients hospitalized with COVID-19 infection.3
A suspected case according to the WHO is a patient ‘with severe acute respiratory infection (fever, cough and requiring admission to hospital), and with no other etiology that fully explains the clinical presentation and at least one of the following: a history of travel to or residence in the city of Wuhan, Hubei Province, China in the 14 days prior to symptom onset, or the patient is a health care worker who has been working in an environment where severe acute respiratory infections of unknown etiology are being cared for’.11 A confirmed case is ‘a person with laboratory confirmation of COVID-19 infection, irrespective of clinical signs and symptoms’.11 On February 13 2020 the Hubei National Health Commission said it would include cases confirmed by clinical diagnosis using CT scans in addition to rt-PCR, adding several thousand new cases to the total count.
Diagnosis of MERS by the WHO is defined initially as patients presenting with a fever, cough and hospitalization with suspicion of lower respiratory tract involvement.12 Patient history was obtained upon hospitalization and prominent considerations for diagnosis involved a history of contact with probable or confirmed cases of the illness, or a reported history of travel or residence within the Arabian Peninsula. Severe cases were subjected to laboratory testing.13 Similar to COVID-19, RT-PCR was used for diagnosis. Additional serum tests for antibodies of the virus were developed. In Saudi Arabia, a clinical trial revealed that a combination of lopinavir–ritonavir and interferon beta-1b was shown to be effective among MERS cases.14 Additionally, a broad-spectrum antiviral nucleotide prodrug named remdesivir presented potent efficacy for the treatment of MERS coronavirus and SARS coronavirus in preclinical studies.15,16
A patient was considered to have laboratory-confirmed SARS if there was a positive RT-PCR result from two or more clinical specimens, either from different sites or tested in different laboratories, obtained from patients before or after death, or if there was seroconversion by enzyme-linked immunosorbent assay, indirect fluorescent antibody test or neutralization assay.17 Similar to MERS, serologic testing for IgG antibodies was developed for SARS coronavirus. Treatment of SARS involved combination therapy of lopinavir and ritonavir and was associated with substantial clinical benefit with fewer adverse clinical outcomes.18 A broad-spectrum antiviral nucleotide prodrug named remdesivir presented potent efficacy for the treatment of MERS coronavirus and SARS coronavirus in preclinical studies.15,16
There are several similarities between these viruses in their diagnosis and treatment. All three viruses are definitively diagnosed by utilizing cell cultures of respiratory fluids, serum antibody analysis or RT-PCR analysis of respiratory fluids from patients. All three viruses cause pneumonia, and radiography of the lungs is an important diagnostic tool for preliminary and broad identification of the severity of the disease. These viruses are similarly treated with antiviral therapies, although no specific antiviral therapy has yet been approved for COVID-19, with clinical trials underway. The major difference between COVID-19 and its predecessors is that this virus rarely produces runny noses or gastrointestinal symptoms in those infected, which are commonplace in MERS and SARS cases.3
Mode of transmission
There is limited knowledge regarding the transmission of COVID-19. Transmission has been confirmed to occur from human to human, and it is thought to be spread through respiratory droplets from coughs or sneezes.3 Primary cases of COVID-19 have been traced back to the Huanan seafood market, with secondary cases occurring at hospitals among nurses and physicians who had extensive contact with COVID-19 patients. Furthermore, several individuals who did not have direct contact with the Huanan seafood market were diagnosed with the disease.
MERS is also transmitted from close person-to-person contact (primarily in health care settings during the symptomatic phase of the disease), although instances of this transmission were significantly less during the height of the MERS epidemic. The transmission occurs through respiratory secretions from coughing and sneezing, whereas primary cases of the virus have been traced to close contact with infected dromedary camels, the animals identified as the reservoir host for MERS.19
Similarly, the transmission of SARS occurred during close person-to-person contact, via respiratory droplets from sneezing or coughing at a rapid rate, although not as quickly as the current outbreak of COVID-19. Furthermore, fomites, fecal transmission and handling of animals (killing, selling or preparing wild animals) were less common methods of transmission.20
The modes of transmission, although still in part unclear regarding COVID-19, are thought to be the same mechanism for all three viruses. Infection via respiratory droplets or secretions of infected individuals are thought to be the predominant mode of transmission from human to human. The spread of infection for the current outbreak is occurring more rapidly than in the SARS epidemic. Rates of human-to-human transmission were generally lower for MERS, possibly in part due to the higher case fatality ratio (CFR) among those diagnosed with the disease.
Age, sex morbidity and mortality rate
The initial report on the first 41 cases of the COVID-19 outbreak showed that most patients infected with COVID-19 were males [30 [(73%) of 41], with less than half possessing underlying comorbidities [13 (32%)] which included diabetes, hypertension and cardiovascular disease.3 The median age of cases was 49.0 years (Inter Quartile Range 41.0–58.0). Of the initial 41 patients infected, 27 (66%) had been directly exposed to the Huanan seafood market and the CFR was nearly 2%.3 The CFR has remained at 2% since the start of the epidemic.
In contrast, MERS has a much higher CFR (35%), and due to the severity of the illness it often necessitates mechanical ventilation (in 50–89% of cases).21 According to a study outlining the risk of mortality and severity of MERS cases from 2012–15, the mean age of all patients was 50 years (SD: 18), over half of the cases (51.1%) reported underlying comorbidities, whereas 7.6% reported direct contact with a camel.22 Cases were predominantly male (66.6%) and from Saudi Arabia.22 Overall, ∼35% of all patients who were diagnosed with MERS have died. The CFR was higher in Saudi Arabia (42%), whereas South Korea reported 19% with a range from 7% among younger age groups to 40% among older adults (≥60 years).23 Older age and underlying comorbidities were identified as the predominant risk factors for progression of severe MERS.22
The SARS virus had an overall CFR of 11%.24 Women represented 55.7% of those diagnosed with SARS but had a lower CFR than men (13.2 and 22.3%, respectively).24 About 49% of cases were <40 years old with a CFR of 3%, whereas 21.5% of cases were >59 years with the highest CFR among those >59 years (54.5%). Nearly a quarter of cases (23.1%) were healthcare workers with the lowest CFR (2%).24
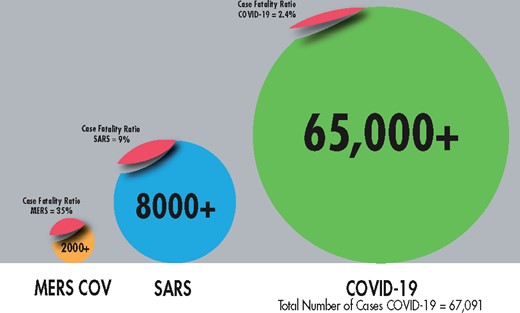
The CFRs across the three viruses range from 2 to 35% (see Figure 2), with the highest among MERS cases and the lowest among the current outbreak, although it is important to note that the CFR for COVID-19 should be interpreted cautiously as the outbreak is still ongoing, and it is hypothesized that many cases are yet to be confirmed, due to a lack of RT-PCR kits in China. It is too early for comparisons to be made between the morbidity and mortality rates of the first two coronaviruses with the current epidemic.
Examining relationships between coronaviruses overall and by country, 15 February 2020. Source country: MERS (Middle East respiratory syndrome), Saudi Arabia; SARS (severe acute respiratory syndrome), Hong Kong (China); and COVID-19 (novel coronavirus), China.2
Infographic comparison of the three major beta coronaviruses, 10 February 2020.
Prevention of zoonotic transmission
Risk factors for COVID-19 are still largely unknown, however, it is believed that the virus was transmitted to humans via contaminated live animals (snakes, civet cats). All three beta coronaviruses emerged via zoonotic transmission. Risk factors for zoonotic transmission of SARS and MERS were direct contact with infected animals. The suspected reservoir hosts are currently believed to be bats, similar to the SARS epidemic. The focal point of the epidemic is the Huanan seafood market. SARS was also hypothesized to have arisen from one of these types of markets. These commonalities may signal the need for closure of these wholesale markets in China. China has a long history of live-animal markets considered vital to communities across the country. As such, it is unlikely that these markets will be closed permanently, although their closure would be the strongest deterrent to another zoonotic disease outbreak. Re-opening of these markets should be under strict purview of the CCDCP, and appropriate measures should be taken to ensure health and hygiene protocols that limit live-animal and human contact are used. Surveillance of these markets may be vital for controlling the spread of zoonotic diseases. Surveillance activities may be similar to that of novel influenza viruses undertaken by the US Center for Disease Control and Prevention.
The three viruses are similar in zoonotic transmission from infected animals to humans. The MERS virus reservoir host is the dromedary camel, the SARS reservoir hosts are likely bats. It is still unclear whether COVID-19 was zoonotically transmitted from an infected civet cat, snake or other animal at the Huanan seafood market.
Challenges to control
Several challenges have been identified in preventing the spread of COVID-19. Among them are limited coordinated efforts among stakeholders with few policies in place for inter-sectoral collaboration, a lack of medical supplies (shortages of masks, goggles) and laboratory facilities for assessment of the disease. Additionally, many cases may have been asymptomatic; so it is difficult to predict when the epidemic will peak and introduces further difficulty in the detection of cases. A recent correspondence to the New England Journal of Medicine documented an asymptomatic contact in Germany.25 In order to contain the virus, Chinese authorities prevented travel to, from and within the city of Wuhan on 23 January, as airline and railway departures were suspended. Between the 23 and 25 January, travel restrictions were implemented in 18 additional cities, affecting nearly 60 million people. The orders issued by Beijing were significant, but late in coming: the first official case of the virus was confirmed almost 2 months previously (8 December 2019) and the length of time it took for China's state-controlled media to reveal the nature of the illness was too great. This can be attributed to a failure of proper risk assessment and management by the Chinese government and health ministry. By the time travel was suspended, however, millions of Chinese citizens had passed through the affected region, unaware of the risks involved.
SARS too, spread globally, due to many of the clinical features of the disease being unknown early in the course of the outbreak, and the significant variation in clinical infection control among South East Asian countries. Similar to the current situation in China, there were inadequate supplies of protective gear for the general population, which, had they been available, could have slowed the spread of the illness. SARS signs and symptoms presented rapidly, and health and hospital authorities were ill-prepared. This combined with insufficient communication of the Chinese government with the public led to panic. Additionally, the lack of infrastructure such as infectious disease hospitals in China added complexity to its control. The pandemic cost the global economy an estimated $30–$100 billion.26
MERS on the other hand, did not spread globally rapidly, in part due to the lower risk of human-to-human transmission of the virus. Asymptomatic cases also provided an extra layer of complexity in the control of the disease. The biggest threat to the eradication of the disease is the variability and inadequacy of infection control in the region most heavily impacted by the virus (the Middle East). These inadequate infection control measures included a lack of physical barriers between patients, lack of negative pressure rooms and overall non-adherence to infection protocols such as proper hand hygiene and sanitation methods.27
Parallels can be drawn between SARS and COVID-19 which can partially be attributed to their origins in China. Similar conditions led to the explosive spread of these viruses, including exposure to live animals at open markets, overcrowded conditions, lack of health infrastructure and lack of transparency between government officials and the general population. MERS and COVID-19 are also similar in that cases can remain asymptomatic while still spreading the disease.25 These viruses all erupted with no specific vaccine or treatment recommended. This is hampering efforts by public health and medical practitioners to limit the spread of COVID-19.
Lessons Learned
Healthcare worker infections
Healthcare workers (HCWs) were infected at high rates during the MERS and SARS outbreaks, with 18.6% of MERS cases occurring in HCWs and 21% of SARS cases occurring in HCWs.28,29 HCWs were infected, in part, through the use of nebulizers, endotracheal suction and intubation, cardiopulmonary resuscitation, nasogastric feeding and high flow-rates of oxygen. The high risk presented by these procedures has implications for medical practice and organization of hospital care during the current infectious disease outbreak. The capacity of COVID-19 to infect healthcare workers has been confirmed, although comparisons with MERS and SARS cannot yet be made.
Timely sharing of information
Inadequate risk assessment regarding the urgency of the situation and limited reporting on the virus within China has, in part, led to the rapid spread of COVID-19 throughout mainland China and into nearby and distant countries.
Collaboration between organizations and governments
Collaboration between governmental agencies and outside organizations (i.e. the CDC and WHO) can prove key to combatting the spread of an epidemic through risk communication and disseminating public health information, as evidenced by the rapid response to the MERS outbreak in South Korea in 2015.30 With respect to the current outbreak, the Chinese Ministry of Health shared the genetic sequencing of the COVID-19 virus 8 days after isolating this virus (10 January), which provided other countries with the ability to diagnose the virus quickly utilizing rapid testing methods, although government transparency at the start of the outbreak was not ideal. It took the Chinese government from 8 December, the first case of the virus, to the 3 January for the initiation of emergency monitoring, case investigation and investigation of the seafood market. Additionally, it took the government from 31 December, when the Wuhan Health Commission announced the outbreak, until 8 January for the government to publicly declare the novel coronavirus was the cause.
Spread of the disease
Compared with SARS and MERS, COVID-19 has spread more rapidly, due in part to increased globalization and the focus of the epidemic. Wuhan, China is a large hub connecting the North, South, East and West of China via railways and a major international airport. The availability of connecting flights, the timing of the outbreak during the Chinese (Lunar) New Year, and the massive rail transit hub located in Wuhan has enabled the virus to perforate throughout China, and eventually, globally (see Figure 3).31
Discussion
The new outbreak of respiratory illness caused by a novel coronavirus termed ‘COVID-19’ has emerged as a serious global public health concern.32 The illness was first announced on 31 December 2019,33,34 and the rapid spread of the virus is fueling fears of a global pandemic.35 During the initial period of the outbreak, from 8 December to 21 January 425 cases were identified, with a growing number of cases not linked to the Huanan seafood market.1 The number of cases reported and documented from the declaration of the initial outbreak, to date, has grown exponentially. At the time of writing, there are now 42 820 cases of COVID-19, with 1014 deaths, and the virus has spread to >26 countries.
COVID-19 is a new strain of coronavirus not previously identified in humans.36 Coronaviruses are zoonotic and are a large family of viruses that cause illness ranging from the common cold to more severe diseases, such as MERS and SARS.21 Initially, many of the patients in the outbreak in Wuhan reported some link to a large seafood and live-animal market, suggesting zoonotic transmission. Scientists also believe that an animal source is ‘the most likely primary source’, and human-to-human transmission has occurred, with growing numbers of cases reportedly without exposure to animal markets.3 Early in the outbreak, a top Chinese government-appointed expert stated a mysterious respiratory illness had killed at least four people with evidence of human-to-human transmission, heightening public concern.36,37 It is likely that person-to-person spread will continue to occur, and similarities can be drawn more closely to SARS than MERS, because of the virus’ rapid rate of infection.38 When person-to-person spread occurred with SARS and MERS, it is thought to have happened via respiratory droplets produced when an infected person coughs or sneezes, similar to how other respiratory pathogens spread.12,29 This is the same hypothesized mechanism of transmission for COVID-19. The spread of MERS and SARS between people has generally occurred between close contacts, similar to the current epidemic in China. According to the WHO, common signs of COVID-19 infection include respiratory symptoms, fever, cough, shortness of breath and breathing difficulties. Serious cases can lead to pneumonia, severe acute respiratory syndrome, kidney failure and death.39 The WHO has advised avoidance of ‘unprotected’ contact with live animals, to thoroughly cook meat and eggs, and avoiding close contact with anyone with cold or flu-like symptoms. Additionally, the CDC issued a travel statement to avoid all non-essential travel to China (Level 3 Travel Notice).40 Control measures have been put into effect, although the timing of these measures and the relatively rapid spread of the current virus suggests that lessons from the previous SARS and MERS epidemics were not heeded.
The surge in infections is alarming due to increased international travel around the Lunar New Year (25 January) and Chinese business ties across the globe. Researchers should critically review the virus’ genome sequence to ascertain the presence of human-to-human transmission, incubation period, modes of transmission, the common source of exposure and the presence of asymptomatic or mildly symptomatic cases that go undetected. Risk assessment is badly needed to control the impact of the virus. This risk assessment should include an evaluation of the current standard of epidemiologic surveillance and identifying the risk factors for human-to-human transmission from asymptomatic cases.
Since there is no specific treatment for coronaviruses,39 there is an urgent need for global surveillance of humans infected with COVID-19. The combined role of internet of things (IoT) and related technologies can play a vital role in preventing the spread of zoonotic infectious diseases. Smart disease surveillance systems could enable simultaneous reporting and monitoring, end-to-end connectivity, data assortment and analysis, tracking and alerts. Remote medical assistance should also be adopted to detect and control zoonotic infectious disease outbreaks.
Airport authorities around the globe must take precautionary measures to reduce the risk of importation of COVID-19. Urgent actions such as screening air passengers traveling from China are needed to contain the spread of suspected COVID-19 cases. Patients with symptoms of respiratory diseases must be reported to the authorities. After further investigation, travelers that are symptomatic or fit the case definition for the novel coronavirus need to be sent to local hospitals for further management. However, this may prove difficult due to the asymptomatic nature of some cases. Wuhan alone has connections with more than 60 overseas destinations through its international airport, whereas Beijing, Shanghai and Shenzhen, all of which have reported cases, have hundreds more. Furthermore, airport authorities should also display alerts on the signs and symptoms of the virus, and preventive measures should be taken by travelers around the globe.
In order to halt the spread of the COVID-19 outbreak, affected countries should look to past successes and failures of beta-coronavirus spread. Lessons learned from the MERS and SARS outbreaks can provide valuable insight into how to handle the current epidemic. These include proper hand hygiene, isolation of infected individuals in properly ventilated hospitals (negative pressure rooms), isolation of individuals with suspected symptoms or fever, and preventing direct contact with suspected animal reservoir hosts. Unfortunately, some lessons were not heeded. Transparency was lacking between government officials and the public, which in turn led to the rapid spread of the virus. In contrast, the rapid sharing of the genetic sequencing of the virus has led to faster diagnoses on a global scale. The Chinese government was also able to quickly build hospitals in order to house the infected, although these steps came too late to prevent the spread. The top concern of health officials now should be halting the spread of the infection. Thus far, >40 000 people have been infected, with no end in sight to the epidemic. Basic epidemiological parameters of patients including person, place and time of diagnosis, as well as clinical signs and symptoms, outcome of the infection, severity, exposures and travel histories must be ascertained for each case. The role that live-animal markets have played in the SARS and COVID-19 epidemics highlight the need for a paradigm shift in China away from their use. Closure or suspension of these markets would be the most conservative approach to preventing zoonotic transmission, however China has a long history of live markets, and their permanent ban is unlikely. In this case, the use of proper hygiene and protocols for limiting animal-to-human contact would be ideal, as well as increased epidemiologic surveillance and monitoring. More research should be carried out on the development of effective methods to provide early and timely detection of such diseases. These methods have the potential to reduce morbidity and mortality. Future research should attempt to address the uses and implications of IoT technologies for mapping the spread of infection. Necessary effective measures need to be taken to avoid the unpredictable risk of continuing outbreaks in China and the possibility of a local outbreak turning into a global pandemic.
Funding
Part of Dr. Wenyi Zhang's time was sponsored by The National Key Research and Development Project of China (2019YFC1200500; 2019YFC1200501)
The authors disclose no other sources of funding.
Acknowledgements
The authors would like to acknowledge Mr Ariel Peeri, for his development of the infographic in Figure 2.
Author Contributions
All authors conceptualized and designed the study, N.C.P. and U.H. drafted the manuscript and made final revisions, and all authors critically revised, read and approved the final manuscript.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
WHO. MERS-CoV. https://www.who.int/emergencies/mers-cov/en/ (2 February 2020, date last accessed
WHO. Clinical Management of Severe Acute Respiratory Infection when Novel Coronavirus (2019-nCoV) Infection is Suspected.
World Health Organization (WHO).
World Health Organization (WHO). Novel Coronavirus 2019.
World Health Organization (WHO). Novel Coronavirus (2019-nCoV) situation reports.
BBC NEWS. New China Virus: Warning against Cover-up as Number of Cases Jumps.
Centers for Disease Control and Prevention C. 2019 Novel Coronavirus (2019-nCoV), Wuhan, China.
WORLD Health Organization (WHO). Coronavirus.
CDC. CDC Advises Travelers to Avoid All Nonessential Travel to China. https://www.cdc.gov/media/releases/2020/s0128-travelers-avoid-china.html (2 April 2020, date last accessed).