Responses of Phytoplankton Communities in Selected Eutrophic Lakes to Variable Weather Conditions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
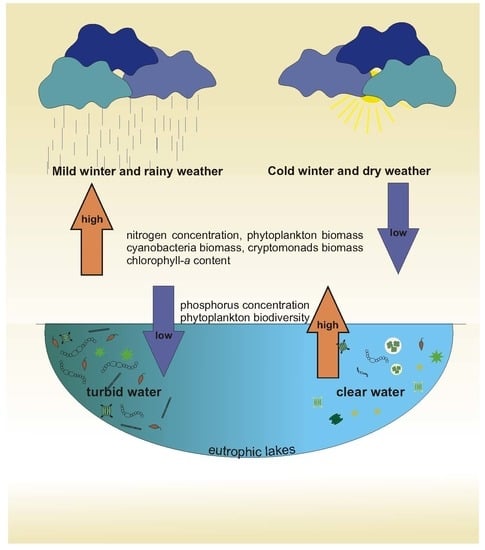
- The changes in weather conditions, defined as fluctuations in air temperature and total precipitation, significantly affected the functioning of the aquatic ecosystem in the studied lakes in the temperate zone.
- The occurrence of a cold or mild winter affected both the abiotic and biotic parameters of the studied lakes during the vegetative growth season.
- The content of soluble and total nitrogen, concentration of chlorophyll a, total phytoplankton biomass, and biomasses of Cyanobacteria and Cryptophyceae were significantly higher in the vegetative growth seasons following a mild winter, whereas the content of soluble and total phosphorus and phytoplankton biodiversity were significantly lower in these years.
- All of the features described in this study showed how sensitive lake ecosystems are to climatic fluctuations. The functioning of the lake ecosystems during the vegetative growth season was not only affected by climatic variability during the season itself, but also as a consequence of changes that took place earlier. Hence, climatic effects on lake ecosystems should always be considered over an extended period of time.
- Climate warming coupled with frequent mild winters could promote the mass development of phytoplankton blooms, which could indirectly affect the biodiversity of phytoplankton communities in lakes in the temperate zone.
Author Contributions
Funding
Conflicts of Interest
References
- George, D.G. The Impact of Climate Change on European Lakes; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Crutzen, P.J. Geology of mankind: The Anthropocene. Nature 2002, 415, 23. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Persson, Å.; Deutch, L.; Zalasiewicz, J.; Williams, M.; Richardson, K.; Crumley, C.; Crutzen, P.; Folke, C.; Gordon, L.; et al. The Anthropocene: From Global Change to Planetary Stewardship. Ambio 2011, 40, 739–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beniston, M.; Stephenson, D.B.; Christensen, O.B.; Ferro, C.A.T.; Frei, C.; Goyette, S.; Halsnaes, K.; Holt, T.; Jylhä, K.; Koffi, B.; et al. Future Extreme Events in European Climate: An Exploration of Regional Climate Model Projections. Clim. Chang. 2007, 81, 71–95. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Dokulil, M.T. Predicting summer surface water temperatures for large Austrian lakes in 2050 under climate change scenarios. Hydrobiologia 2014, 731, 19–29. [Google Scholar] [CrossRef]
- O’Reilly, C.M.; Sharma, S.; Gray, D.K.; Hampton, S.E.; Read, J.S.; Rowley, R.J.; Schneider, P.; Lenters, J.D.; McIntyre, P.B.; Kraemer, B.M.; et al. Rapid and highly variable warming of lake surface waters around the globe. Geophys. Res. Lett. 2015, 42, 773–781. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Meilei, M.; Livingstone, D.M. Nonlinear temperature response of lake ice breakup. Geophys. Res. Lett. 2004, 31, L07203. [Google Scholar] [CrossRef]
- Marszelewski, W.; Skowron, R. Ice cover as an indicator of winter air temperature changes: Case study of the Polish Lowland lakes. Hydrol. Sci. J. 2006, 51, 336–349. [Google Scholar] [CrossRef]
- Livingstone, D.M.; Adrian, R.; Blenckner, T.; George, G.; Weyhenmeyer, G.A. Lake ice phenology. In The Impact of Climate Change on European Lakes; George, D.G., Ed.; Aquatic Ecology Series 4; Springer Science+Business Media B.V.: London, UK; New York, NY, USA, 2010; pp. 51–61. [Google Scholar] [CrossRef]
- Leppäranta, M. Modelling the formation and decay of lake ice. In The Impact of Climate Change on European Lakes; George, D.G., Ed.; Aquatic Ecology Series 4; Springer Science+Business Media B.V.: London, UK; New York, NY, USA, 2010; pp. 63–83. [Google Scholar] [CrossRef]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.W.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; De Meester, L.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T. Throwing fuel on the fire: Synergistic effects of excessive nitrogen inputs and global warming on harmful algal blooms. Environ. Sci. Technol. 2010, 44, 7756–7758. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.E.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hessen, D.O.; Andersen, T.; Larsen, S.; Skjelkvåle, B.L.; de Wit, H.E. Nitrogen deposition, catchment productivity, and climate as determinants of lake stoichiometry. Limnol. Oceanogr. 2009, 54, 2520–2528. [Google Scholar] [CrossRef]
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Wetzel, R. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego/San Francisco, CA, USA; New York, NY, USA; Boston, MA, USA; London, UK; Sydney, Australia; Tokyo, Japan, 2001. [Google Scholar]
- Ribeiro, K.F.; Duarte, L.; Crossetti, L.O. Everything is not everywhere: A tale on the biogeography of cyanobacteria. Hydrobiologia 2018, 820, 23–48. [Google Scholar] [CrossRef]
- Johnk, K.D.; Huisman, J.; Sharples, J.P.; Sommeijer, B.; Visser, P.M.; Stroom, J.M. Summer heatwaves promote blooms of harmful cyanobacteria. Glob. Chang. Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Bécares, E.; Costa, L.S.; Van Donk, E.; Hansson, L.-A.; Jeppesen, E.; Kruk, C.; Lacerot, G.; Mazzeo, N.; et al. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Chang. Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Elliott, J.A. Is the future blue-green? A review of the current model predictions of how climate change could affect pelagic freshwater cyanobacteria. Water Res. 2012, 46, 1364–1371. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.S.; Oliver, R.L.; Walsby, A.E. Cyanobacterial dominance: The role of buoyancy regulation in dynamic lake environments. N. Z. J. Mar. Freshw. Res. 1987, 21, 379–390. [Google Scholar] [CrossRef]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharm. 2005, 203, 264–272. [Google Scholar] [CrossRef]
- Nõges, P.; Adrian, R.; Anneville, O.; Arvola, L.; Blenckner, T.; George, G.; Jankowski, T.; Järvinen, M.; Maberly, S.; Padisák, J.; et al. The impact of variations in the climate on seasonal dynamics of phytoplankton. In The Impact of Climate Change on European Lakes; George, D.G., Ed.; Aquatic Ecology Series 4; Springer Science+Business Media B.V.: London, UK; New York, NY, USA, 2010; pp. 253–274. [Google Scholar] [CrossRef]
- Lenard, T. Winter bloom of some motile phytoplankton under ice cover in a mesotrophic lake: Vertical distribution and environmental factors. Oceanol. Hydrobiol. Stud. 2015, 44, 164–171. [Google Scholar] [CrossRef]
- Block, B.D.; Denfeld, B.A.; Stockwell, J.D.; Flaim, G.; Grossart, H.-P.F.; Knoll, L.B.; Maier, D.B.; North, R.L.; Rautio, M.; Rusak, J.A.; et al. The unique methodological challenges of winter limnology. Limnol. Oceanogr. Methods 2019, 17, 42–57. [Google Scholar] [CrossRef]
- Lenard, T.; Wojciechowska, W. Phytoplankton diversity and biomass during winter with and without ice cover in the context of climate change. Pol. J. Ecol. 2013, 61, 739–748. [Google Scholar]
- Hayden, B.; Harrod, C.; Kahilainen, K.K. The effects of winter ice cover on the trophic ecology of whitefish (Coregonus lavaretus L.) in subarctic lakes. Ecol. Freshw. Fish 2013, 22, 192–201. [Google Scholar] [CrossRef]
- Wojciechowska, W.; Lenard, T. Effect of extremely severe winters on under-ice phytoplankton development in a mesotrophic lake (Eastern Poland). Oceanol. Hydrobiol. Stud. 2014, 43, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Hampton, S.E.; Moore, M.V.; Ozersky, T.; Stanley, E.H.; Polashenski, C.M.; Galloway, A.W.E. Heating up a cold subject: Prospects for under-ice plankton research in lakes. J. Plankton Res. 2015, 37, 277–284. [Google Scholar] [CrossRef]
- Kalinowska, K.; Grabowska, M. Autotrophic and heterotrophic plankton under ice in a eutrophic temperate lake. Hydrobiologia 2016, 777, 111–118. [Google Scholar] [CrossRef]
- Özkundakci, D.; Gsell, A.S.; Hintze, T.; Täuscher, H.; Adrian, R. Winter severity determines functional trait composition of phytoplankton in seasonally ice-covered lakes. Glob. Chang. Biol. 2016, 22, 284–298. [Google Scholar] [CrossRef]
- Grosbois, G.; Mariash, H.; Schneider, T.; Rautio, M. Under-ice availability of phytoplankton lipids is key to freshwater zooplankton winter survival. Sci. Rep. 2017, 7, 11543. [Google Scholar] [CrossRef]
- Hampton, S.E.; Galloway, A.W.E.; Powers, S.M.; Ozersky, T.; Woo, K.H.; Batt, R.D.; Labou, S.G.; O’Reilly, C.M.; Sharma, S.; Lottig, N.R. Ecology under lake ice. Ecol. Lett. 2017, 20, 98–111. [Google Scholar] [CrossRef]
- Glé, C.; Del Amo, Y.; Bec, B.; Sautour, B.; Froidefond, J.-M.; Gohin, F.; Maurer, D.; Plus, M.; Laborde, P.; Chardy, P. Typology of environmental conditions at the onset of winter phytoplankton blooms in a shallow macrotidal coastal ecosystem, Arcachon bay (France). J. Plankton Res. 2007, 29, 999–1014. [Google Scholar] [CrossRef]
- Laugaste, R.; Haberman, J.; Blank, K. Cool winters versus mild winters: Effects on spring plankton in Lake Peipsi. Est. J. Ecol. 2010, 59, 163–183. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Westöö, A.-K.; Willén, E. Increasingly ice-free winters and their effects on water quality in Sweden’s largest lakes. Hydrobiologia 2008, 599, 111–118. [Google Scholar] [CrossRef]
- Lürling, M.; De Senerpont Domis, L.N. Predictability of plankton communities in an unpredictable world. Freshw. Biol. 2013, 58, 455–462. [Google Scholar] [CrossRef]
- Pełechata, A.; Pełechaty, M.; Pukacz, A. Winter temperature and shifts in phytoplankton assemblages in asmall Chara-lake. Aquat. Bot. 2015, 124, 10–18. [Google Scholar] [CrossRef]
- Lenard, T.; Ejankowski, W. Natural water brownification as a shift in the phytoplankton community in a deep hard water lake. Hydrobiologia 2017, 787, 153–166. [Google Scholar] [CrossRef]
- Lürling, M.; Eshetu, F.; Faassen, E.J.; Kosten, S.; Huszar, V.L.M. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol. 2013, 58, 552–559. [Google Scholar] [CrossRef]
- Kondracki, J. Geografia regionalna Polski; Wyd. Naukowe PWN: Warszawa, Poland, 2002. [Google Scholar]
- Ejankowski, W.; Lenard, T. Trophic state of a shallow lake with reduced inflow of surface water. Arch. Environ. Prot. 2014, 40, 3–11. [Google Scholar] [CrossRef]
- Bajkiewicz-Grabowska, E. Assessment of the ecological state of lakes as proposed by the Polish Limnological Society. Limnol. Rev. 2010, 10, 105–116. [Google Scholar] [CrossRef]
- Wilgat, T.; Michalczyk, Z.; Turczyński, M.; Wojciechowski, K. The Łęczna-Włodawa Lakes. Stud. Ośr Dok Fizjogr PAN 1991, 19, 23–140. [Google Scholar]
- Nush, E.A. Comparison of different methods for chlorophyll and pheopigment determination. Arch. Hydrobiol. 1980, 14, 14–36. [Google Scholar]
- Hermanowicz, W.; Dojlido, J.; Dożańska, W.; Koziorowski, B.; Zerbe, J. Fizyczno-Chemiczne Badanie Wody I Ścieków; Wyd. Arkady: Warszawa, Poland, 1999. [Google Scholar]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Kratzer, C.R.; Brezonik, P.L. A Carlson-type trophic state index for nitrogen in Florida lakes. Water Resour. Bull. 1981, 17, 713–715. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervolkommnung der quantitativen Planktonmethodik. Mitt. Int. Ver. Für Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Pielou, E.C. Ecological Diversity; John Wiley & Sons: New York, NY, USA, 1975. [Google Scholar]
- Reynolds, C. The Ecology of Phytoplankton. Ecology, Biodiversity and Conservation; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry, 3rd ed.; W.H. Freeman and Company: New York, NY, USA, 1995. [Google Scholar]
- Havens, K.E. Secondary nitrogen limitation in a subtropical lake impacted by non-point source agricultural pollution. Environ. Pollut. 1995, 89, 241–246. [Google Scholar] [CrossRef]
- Nõges, T.; Nõges, P. The effect of extreme water level decrease on hydrochemistry and phytoplankton in a shallow eutrophic lake. Hydrobiologia 1999, 408/409, 277–283. [Google Scholar] [CrossRef]
- Kleeberg, A.; Freidank, A.; Jöhnk, K. Effects of ice cover on sediment resuspension and phosphorus entrainment in shallow lakes: Combining in situ experiments and wind-wave modeling. Limnol. Oceanogr. 2013, 58, 1819–1833. [Google Scholar] [CrossRef]
- Klimaszyk, P.; Rzymski, P. Surface runoff as a factor determining trophic state of midforest lake. Pol. J. Environ. Stud. 2011, 20, 1203–1210. [Google Scholar]
- Blank, K.; Haberman, J.; Haldna, M.; Laugaste, R. Effect of winter conditions on spring nutrient concentrations and plankton in a large shallow Lake Peipsi (Estonia/Russia). Aquat. Ecol. 2009, 43, 745–753. [Google Scholar] [CrossRef]
- Sickman, J.O.; Leydecker, A.; Chang, C.C.Y.; Kendall, C.; Melack, J.M.; Lucero, D.M.; Schimel, J. Mechanisms underlying export of N from high-elevation catchments during seasonal transitions. Biogeochemistry 2003, 64, 1–24. [Google Scholar] [CrossRef]
- Rasconi, S.; Winter, K.; Kainz, M.J. Temperature increase and fluctuation induce phytoplankton biodiversity loss—Evidence from a multi-seasonal mesocosm experiment. Ecol. Evol. 2017, 7, 2936–2946. [Google Scholar] [CrossRef] [PubMed]
- Havens, K.E.; Paerl, H.; Phlips, E.J.; Zhu, M.; Beaver, J.R.; Srifa, A. Extreme weather events and climate variability provide a lens into how shallow lakes may respond to climate change. Water 2016, 8, 229. [Google Scholar] [CrossRef]
- Jankowiak, J.; Hattenrath-Lehmann, T.; Kramer, B.J.; Ladds, M.; Gobler, C.J. Deciphering the effects of nitrogen, phosphorus, and temperature on cyanobacterial bloom intensification, diversity, and toxicity in western Lake Erie. Limnol. Oceanogr. 2019, 1–24. [Google Scholar] [CrossRef]
- Lepistö, L.; Saura, M. Effects of forest fertilization on phytoplankton in boreal brown-water lake. Boreal Environ. Res. 1998, 3, 33–43. [Google Scholar]
- Cottingham, K.L.; Ewing, H.A.; Greer, M.L.; Carey, C.C.; Weathers, K.C. Cyanobacteria as biological drivers of lake nitrogen and phosphorus cycling. Ecosphere 2015, 6, 1–19. [Google Scholar] [CrossRef]










| Lake | Coordinates | Area (ha) | Length (m) | Mean Width (m) | Depth (m) | Volume (103 m3) | Catchment Area (ha) | |
|---|---|---|---|---|---|---|---|---|
| Max | Mean | |||||||
| Głębokie | 51°28′34″ N 22°55′23″ E | 20.5 | 585 | 350 | 7.1 | 3.4 | 689 | 173.8 |
| Gumienek | 51°30′14″ N 22°56′20″ E | 8.1 | 376 | 215 | 7.8 | 3.8 | 307 | 21.5 |
| Czarne | 51°29′08″ N 22°56′34″ E | 24.8 | 596 | 416 | 10.3 | 3.7 | 915 | Earth dyke |
| Maśluchowskie | 51°28′03″ N 22°56′43″ E | 26.7 | 861 | 310 | 9.4 | 4.6 | 1231 | 113.9 |
| Parameter | CW | MW | df | F-test | ||||
|---|---|---|---|---|---|---|---|---|
| Group of Lakes | Group of Lakes | |||||||
| A | B | All Lakes | A | B | All Lakes | |||
| Chl-a (μg L−1) | 29.39 (±15.0) | 13.79 (±10.9) | 21.51 (±15.2) | 36.05 (±16.3) | 20.62 (±21.7) | 28.34 (±20.6) | 1 | 6.95 ** |
| Total Biomass (mg L−1) | 11.79 (±6.3) | 5.55 (±3.6) | 8.64 (±6.0) | 13.51 (±6.5) | 8.49 (±8.2) | 11.01 (±7.7) | 1 | 6.47 * |
| Cyanobacteria (mg L−1) | 4.43 (±3.4) | 1.39 (±2.1) | 2.89 (±3.2) | 5.54 (±5.0) | 2.52 (±5.1) | 4.03 (±5.2) | 1 | 4.47 * |
| Cryptophyceae (mg L−1) | 2.18 (±1.2) | 0.78 (±0.7) | 1.47 (±1.2) | 2.92 (±1.5) | 1.35 (±1.2) | 2.14 (±1.6) | 1 | 14.65 *** |
| Euglenophyta (mg L−1) | 0.32 (±0.3) | 0.03 (±0.0) | 0.17 (±0.2) | 0.29 (±0.3) | 0.05 (±0.0) | 0.17 (±0.2) | 1 | 0.02 |
| Dinophyceae (mg L−1) | 0.87 (±0.8) | 0.31 (±0.5) | 0.59 (±0.7) | 0.79 (±0.9) | 0.44 (±0.9) | 0.61 (±0.9) | 1 | 0.15 |
| Chrysophyceae (mg L−1) | 0.11 (±0.2) | 0.06 (±0.1) | 0.09 (±0.2) | 0.14 (±0.3) | 0.05 (±0.0) | 0.09 (±0.2) | 1 | 0.02 |
| Bacillariophyceae (mg L−1) | 0.51 (±0.6) | 0.26 (±0.4) | 0.39 (±0.5) | 0.47 (±0.6) | 0.19 (±0.3) | 0.33 (±0.5) | 1 | 0.96 |
| Chlorophyta (mg L−1) | 3.38 (±3.0) | 2.71 (±1.8) | 3.05 (±2.5) | 3.38 (±2.4) | 3.88 (±2.9) | 3.63 (±2.7) | 1 | 2.03 |
| Species richness | 41.6 (±8.1) | 32.0 (±7.7) | 36.7 (±9.2) | 34.4 (±9.3) | 26.4 (±8.3) | 30.4 (±9.7) | 1 | 22.45 *** |
| Shannon-Wiener diversity index (H’) | 2.05 (±0.6) | 2.15 (±0.6) | 2.10 (±0.6) | 1.92 (±0.6) | 1.74 (±0.6) | 1.83 (±0.6) | 1 | 7.39 ** |
| Pielou’s evenness (J) | 0.55 (±0.1) | 0.61 (±0.1) | 0.59 (±0.1) | 0.54 (±0.1) | 0.53 (±0.1) | 0.53 (±0.1) | 1 | 3.94 * |
| TSI (TN) | 63.3 (±7.7) | 62.3 (±7.7) | 62.8 (±7.7) | 73.9 (±5.9) | 71.1 (±6.9) | 72.5 (±6.5) | 1 | 68.01 *** |
| TSI (TP) | 72.7 (±11.6) | 69.3 (±13.6) | 71 (±12.7) | 59.7 (±4.9) | 54.1 (±7.2) | 56.9 (±6.7) | 1 | 68.59 *** |
| TSI (CHL) | 62.6 (±4.8) | 54.0 (±6.6) | 58.3 (±7.3) | 64.8 (±4.4) | 56.9 (±7.7) | 60.9 (±7.4) | 1 | 7.03 ** |
| TSI (SD) | 56.8 (±3.2) | 46.3 (±5.2) | 51.5 (±6.8) | 58.1 (±4.9) | 48.2 (±6.9) | 53.2 (±7.8) | 1 | 3.94 * |
| Kd (m−1) | 1.67 (±0.9) | 0.97 (±0.4) | 1.32 (±0.7) | 1.86 (±0.7) | 1.25 (±0.8) | 1.56 (±0.8) | 1 | 4.46 * |
| SD (m) | 1.28 (±0.3) | 2.75 (±0.9) | 2.03 (±1.0) | 1.21 (±0.4) | 2.51 (±1.1) | 1.86 (±1.0) | 1 | 1.76 |
| Water temperature (°C) | 22.16 (±2.9) | 21.74 (±2.7) | 21.95 (±2.8) | 21.5 (±2.6) | 21.0 (±2.5) | 21.2 (±2.6) | 1 | 2.71 |
| EC (μS cm−1) | 266.1 (±68.4) | 186.8 (±59.6) | 226.1 (±75.2) | 271.9 (±81.2) | 195.8 (±64.9) | 233.9 (±82.4) | 1 | 0.46 |
| pH | 7.75 (±0.3) | 8.01 (±0.3) | 7.89 (±0.3) | 7.88 (±0.3) | 7.96 (±0.3) | 7.93 (±0.3) | 1 | 0.03 |
| TN (mg L−1) | 2.09 (±0.9) | 1.94 (±0.8) | 2.02 (±0.9) | 4.12 (±1.3) | 3.49 (±1.5) | 3.80 (±1.4) | 1 | 96.88 *** |
| TP (mg L−1) | 0.16 (±0.1) | 0.15 (±0.2) | 0.16 (±0.2) | 0.05 (±0.0) | 0.04 (±0.0) | 0.04 (±0.0) | 1 | 30.66 *** |
| P-PO4 (mg L−1) | 0.03 (±0.0) | 0.02 (±0.0) | 0.03 (±0.0) | 0.01 (±0.0) | 0.01 (±0.0) | 0.01 (±0.0) | 1 | 23.25 *** |
| DIN (mg L−1) | 0.53 (±0.4) | 0.61 (±0.5) | 0.58 (±0.5) | 2.54 (±1.2) | 2.09 (±1.1) | 2.18 (±1.1) | 1 | 144.78 *** |
| DIN:TN | 0.28 (±0.2) | 0.32 (±0.2) | 0.30 (±0.2) | 0.52 (±0.2) | 0.58 (±0.2) | 0.55 (±0.2) | 1 | 64.19 *** |
| P-PO4:TP | 0.24 (±0.2) | 0.29 (±0.2) | 0.27 (±0.2) | 0.28 (±0.2) | 0.37 (±0.2) | 0.33 (±0.2) | 1 | 3.4 |
| TN:TP | 25.3 (±25.2) | 34.0 (±30.4) | 29.7 (±28.2) | 94.9 (±49.9) | 110.2 (±55.1) | 102.6 (±52.7) | 1 | 129.17 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenard, T.; Ejankowski, W.; Poniewozik, M. Responses of Phytoplankton Communities in Selected Eutrophic Lakes to Variable Weather Conditions. Water 2019, 11, 1207. https://doi.org/10.3390/w11061207
Lenard T, Ejankowski W, Poniewozik M. Responses of Phytoplankton Communities in Selected Eutrophic Lakes to Variable Weather Conditions. Water. 2019; 11(6):1207. https://doi.org/10.3390/w11061207
Chicago/Turabian StyleLenard, Tomasz, Wojciech Ejankowski, and Małgorzata Poniewozik. 2019. "Responses of Phytoplankton Communities in Selected Eutrophic Lakes to Variable Weather Conditions" Water 11, no. 6: 1207. https://doi.org/10.3390/w11061207